The chemisorption thermodynamics of O2 and H2O on AFM UO2 surfaces unraveled by DFT + U-D3 study
IF 6.9
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Unraveling the adsorption mechanism and thermodynamics of O2 and H2O on uranium dioxide surfaces is critical for the nuclear fuel storage and uranium corrosion. Based on the first-principles DFT + U-D3 calculations, we carefully test the effect of antiferromagnetic order arrangements on the thermodynamic stability of UO2 surfaces and propose the 1k AFM surface computational model. The chemisorption states of O2 and H2O on UO2(111) surface, suggested by previous experiments, are accurately calculated for the first time. The adsorption properties of O2 and H2O on UO2(111) and (110) surfaces are discussed in detail to reveal the different interaction mechanisms. Combined with ab initio atomistic thermodynamics method, we systematically calculate the chemisorption phase diagram and isotherm of O2 and H2O on UO2 surfaces. Due to the different intermolecular interactions, the monolayer and multilayer adsorption models are identified for O2 and H2O, respectively. This study has comprehensively revealed the different adsorption mechanisms of O2 and H2O on UO2 surfaces, bridging the electronic structure calculations to the interpretation of experimental results and providing a solid foundation for future theoretical studies of uranium corrosion mechanism in humid air.
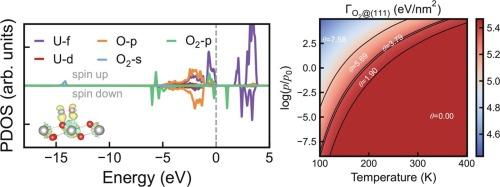

用DFT + U-D3研究了O2和H2O在AFM UO2表面的化学吸附热力学
揭示O2和H2O在二氧化铀表面的吸附机理和热力学对核燃料储存和铀腐蚀具有重要意义。基于第一性原理DFT + U-D3计算,我们仔细测试了反铁磁有序排列对UO2表面热力学稳定性的影响,并提出了1k AFM表面计算模型。本文首次精确计算了前人实验提出的UO2(111)表面O2和H2O的化学吸附态。详细讨论了UO2(111)和UO2(110)表面对O2和H2O的吸附特性,揭示了不同的相互作用机理。结合从头算原子热力学方法,系统地计算了O2和H2O在UO2表面的化学吸附相图和等温线。由于分子间相互作用的不同,分别确定了O2和H2O的单层和多层吸附模型。本研究全面揭示了O2和H2O在UO2表面的不同吸附机理,将电子结构计算与实验结果的解释联系起来,为今后铀在潮湿空气中的腐蚀机理的理论研究提供了坚实的基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Applied Surface Science
工程技术-材料科学:膜
CiteScore
12.50
自引率
7.50%
发文量
3393
审稿时长
67 days
期刊介绍:
Applied Surface Science covers topics contributing to a better understanding of surfaces, interfaces, nanostructures and their applications. The journal is concerned with scientific research on the atomic and molecular level of material properties determined with specific surface analytical techniques and/or computational methods, as well as the processing of such structures.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


