Scalable Co–Ni Mixed MOF Featuring Dual Functional Sites for C2H2 Separation with Excellent Shaping Performance
IF 4.7
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
The purification of C2H2 from gas streams containing CO2 persists as a formidable challenge in petrochemical manufacturing, primarily attributed to the identical kinetic diameters and the limitations of conventional separation methods. Herein, we report a scalable Co–Ni mixed metal–organic framework (MOF), Co(bpy)[Ni(CN)4], featuring dual functional sites, namely, unsaturated Ni2+ open metal sites (OMS) and Lewis basic nitrogen atoms, for efficient C2H2 capture. The framework exhibits a good C2H2 uptake of 51.5 cm3 g–1 at 298 K and 1 bar, surpassing that of many popular MOFs. Synergistic interactions between C2H2 and the dual sites, as revealed by DFT calculations, enable excellent IAST selectivity for equimolar C2H2/CO2 (5.9) and C2H2/CH4 (26.3) mixtures. Dynamic breakthrough experiments confirm robust separation performance under humid conditions, across temperatures (278 to 313 K), and over multiple cycles without degradation. Furthermore, shaping the MOF into pellets using polyvinyl butyraldehyde (PVB) binder retains its porosity and enhances processability, achieving a dynamic C2H2 capture capacity of 37 cm3 g–1 from C2H2/CH4 breakthrough separation. This work demonstrates a scalable, stable, and shapeable MOF with dual functional sites, offering a practical solution for industrial gas separation.
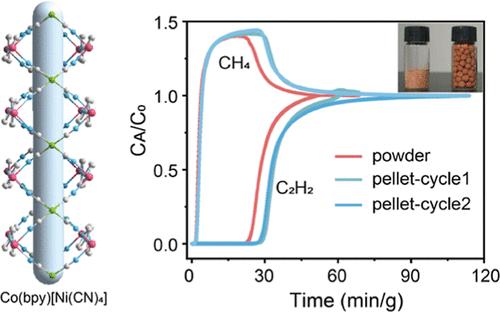
具有双功能位点的可扩展钴镍混合MOF具有优异的成型性能,可用于C2H2分离
从含有二氧化碳的气体流中提纯C2H2一直是石化制造中的一个巨大挑战,主要归因于相同的动力学直径和传统分离方法的局限性。在此,我们报道了一种可扩展的Co - Ni混合金属有机骨架(MOF), Co(bpy)[Ni(CN)4],具有双功能位点,即不饱和Ni2+开放金属位点(OMS)和路易斯碱氮原子,用于有效捕获C2H2。该框架在298 K和1 bar下具有51.5 cm3 g-1的良好C2H2吸收量,超过了许多流行的mof。DFT计算表明,C2H2与双位点之间的协同作用使等摩尔C2H2/CO2(5.9)和C2H2/CH4(26.3)混合物具有良好的IAST选择性。动态突破实验证实了在潮湿条件下,在温度(278至313 K)和多个循环中具有强大的分离性能而不会降解。此外,使用聚乙烯醇丁醛(PVB)粘合剂将MOF塑造成颗粒,保持其孔隙度并提高可加工性,从C2H2/CH4突破分离中实现37 cm3 g-1的动态C2H2捕获能力。这项工作展示了一种具有双重功能位点的可扩展、稳定和可成型的MOF,为工业气体分离提供了实用的解决方案。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


