Insights from the European Medicines Agency on digital health technology derived endpoints
IF 7.5
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
This study evaluates the use of digital health technologies (DHTs) for endpoint measurement in clinical trials, as documented in the Qualification Opinions, Qualification Advice, and Scientific Advice procedures issued by the European Medicines Agency (EMA) between 2013 and 2022. Accelerometers are the most proposed DHTs, followed by glucose monitors and smartphones. Accelerometers are often proposed for nervous system diseases to support mobility measures and objective testing. Most DHTs were proposed for efficacy endpoints. The feedback provided by EMA emphasizes the importance of validation, precision, and a clearly defined context of use. The EMA’s recent action plan further supports advancing DHT methodologies in clinical trials.
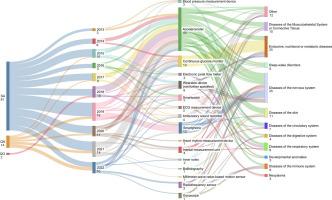
来自欧洲药品管理局关于数字医疗技术衍生端点的见解。
本研究评估了数字健康技术(dht)在临床试验中用于终点测量的使用,如欧洲药品管理局(EMA)在2013年至2022年间发布的资格意见、资格建议和科学建议程序中所记录的。加速度计是建议最多的dht,其次是血糖监测仪和智能手机。加速度计经常被建议用于神经系统疾病,以支持运动测量和客观测试。大多数dht被提出用于疗效终点。EMA提供的反馈强调了验证、准确性和明确定义的使用环境的重要性。EMA最近的行动计划进一步支持在临床试验中推进DHT方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Drug Discovery Today
医学-药学
CiteScore
14.80
自引率
2.70%
发文量
293
审稿时长
6 months
期刊介绍:
Drug Discovery Today delivers informed and highly current reviews for the discovery community. The magazine addresses not only the rapid scientific developments in drug discovery associated technologies but also the management, commercial and regulatory issues that increasingly play a part in how R&D is planned, structured and executed.
Features include comment by international experts, news and analysis of important developments, reviews of key scientific and strategic issues, overviews of recent progress in specific therapeutic areas and conference reports.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


