Strain-engineered Pt-Ni(OH)2 catalyst via a nickel boride intermediated method for high-current-density hydrogen evolution reaction
IF 7.9
3区 材料科学
Q1 GREEN & SUSTAINABLE SCIENCE & TECHNOLOGY
引用次数: 0
Abstract
Efficient hydrogen generation via electrochemical splitting of water at elevated current densities is paramount for its market implementation. Yet, cathodic hydrogen generation, in practical applications, encounters issues such as polarization, which result in energy consumption and sluggishness, thereby limiting its further utilization. In light of this, we have proposed a method for preparing a self-supported Pt-Ni(OH)2 catalyst-(Pt-Ni(OH)2/NF). Specifically, by employing NaBH4 pretreatment on nickel species to form NiB and Ni2B an intermediate, a significant improvement in catalytic performance has been achieved, especially at high current density. The replacement reaction of nickel boride with H2PtCl6 generates rigid strain, leading to the Ni(OH)2 lattice shrinkage. Upon testing, this catalyst requires only a minimal overpotential of 3 mV to achieve a current density of 10 mA cm−2, and it achieved an industrial current of 500 mA cm−2 with only 94 mV overpotential. Moreover, even after a 7-day longevity test at 200 mA cm−2, it still maintained excellent performance.
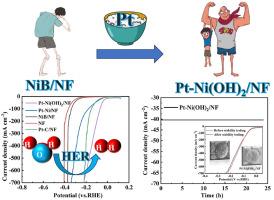
以硼化镍为中间体的应变工程Pt-Ni(OH)2催化剂用于大电流密度析氢反应
在较高的电流密度下,通过电化学分解水来高效制氢对其市场实施至关重要。然而,阴极制氢在实际应用中遇到极化等问题,导致能量消耗和运行缓慢,限制了阴极制氢的进一步利用。鉴于此,我们提出了一种制备自支撑型Pt-Ni(OH)2催化剂的方法-(Pt-Ni(OH)2/NF)。具体而言,采用NaBH4预处理镍种形成NiB和Ni2B中间体,显著提高了催化性能,特别是在高电流密度下。硼化镍与H2PtCl6的取代反应产生刚性应变,导致Ni(OH)2晶格收缩。经过测试,这种催化剂只需要最小的3 mV过电位就可以达到10 mA cm - 2的电流密度,并且它只需要94 mV过电位就可以达到500 mA cm - 2的工业电流。此外,即使在200 mA cm−2下进行了7天的寿命测试,它仍然保持了优异的性能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Materials Today Sustainability
Multiple-
CiteScore
5.80
自引率
6.40%
发文量
174
审稿时长
32 days
期刊介绍:
Materials Today Sustainability is a multi-disciplinary journal covering all aspects of sustainability through materials science.
With a rapidly increasing population with growing demands, materials science has emerged as a critical discipline toward protecting of the environment and ensuring the long term survival of future generations.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


