Hyperwilsol A, a highly modified 2-nor-polycyclic polyprenylated acylphloroglucinol possessing an unusual 6/6/5/5 spirotetracyclic skeleton from Hypericum wilsonii
IF 3.4
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Hypericum species are rich in polycyclic polyprenylated acylphloroglucinols (PPAPs), a class of structurally complex metabolites with diverse biological activities. A phytochemical investigation of the aerial parts of Hypericum wilsonii led to the discovery of four previously undescribed PPAPs, hyperwilsols A–D (1–4), along with 16 known related compounds (5–20). Notably, hyperwilsol A (1) featured a highly modified 6/6/5/5 tetracyclic system based on a rare spiro[1,2-dioxane-3,11'-[3,12]dioxatricyclo[6.4.0.01,5]dodecane] scaffold. The structures with absolute configurations were established by spectroscopic analysis, computer-assisted structure elucidation method, single-crystal X-ray diffraction, and NMR and ECD calculations. Additionally, all isolates were evaluated for their anti-neuroinflammatory and neuroprotective effects. Hyperuralone A (16) and yezo'otogirin A (17) exhibited inhibition of lipopolysaccharide-induced nitric oxide production in BV-2 cells, with their IC50 values of 7.11 ± 0.50 and 10.18 ± 1.75 μM, respectively. Hyperwilsols A–C (1–3), hyperscabin K (9), and hypelodin B (10) showed noticeable neuroprotective activity against H2O2-induced PC-12 cell injury. These findings not only enriched the structural diversity of natural PPAPs but also highlighted their potential in the prevention and treatment of neurodegenerative diseases.
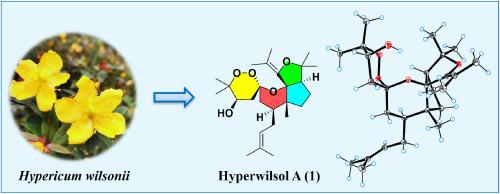
Hyperwilsol A是一种高度改性的2-非多环聚丙烯化酰基间苯三酚,具有罕见的6/6/5/5螺四环骨架
金丝桃属植物富含多环聚丙烯酰化酰基间苯三酚(PPAPs),是一类结构复杂的代谢产物,具有多种生物活性。对金丝桃(Hypericum wilsonii)地上部分的植物化学研究发现了四种以前未描述的PPAPs, hyperwilsols A - d(1-4),以及16种已知的相关化合物(5-20)。值得注意的是,hyperwilsol A(1)具有高度修饰的6/6/5/5四环体系,该体系基于罕见的螺旋[1,2-二氧杂环-3,11'-[3,12]二氧杂环[6.4.0.01,5]十二烷]支架。通过光谱分析、计算机辅助结构解析法、单晶x射线衍射、核磁共振和ECD计算,确定了具有绝对构型的结构。此外,对所有分离物的抗神经炎和神经保护作用进行了评估。Hyperuralone A(16)和yezo’otogirin A(17)对脂多糖诱导的BV-2细胞产生一氧化氮具有抑制作用,其IC50值分别为7.11±0.50 μM和10.18±1.75 μM。Hyperwilsols A-C(1-3)、hyperscabin K(9)和hypelodin B(10)对h2o2诱导的PC-12细胞损伤有明显的神经保护作用。这些发现不仅丰富了天然PPAPs的结构多样性,而且突出了它们在预防和治疗神经退行性疾病方面的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Phytochemistry
生物-植物科学
CiteScore
6.40
自引率
7.90%
发文量
443
审稿时长
39 days
期刊介绍:
Phytochemistry is a leading international journal publishing studies of plant chemistry, biochemistry, molecular biology and genetics, structure and bioactivities of phytochemicals, including ''-omics'' and bioinformatics/computational biology approaches. Phytochemistry is a primary source for papers dealing with phytochemicals, especially reports concerning their biosynthesis, regulation, and biological properties both in planta and as bioactive principles. Articles are published online as soon as possible as Articles-in-Press and in 12 volumes per year. Occasional topic-focussed special issues are published composed of papers from invited authors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


