Stereoisomeric AIE Photosensitizers for Biofilm Inhibition and Host-Directed Elimination of Intracellular Multidrug-Resistant Bacteria
IF 8.2
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
Bacteria can form biofilms on their surfaces or escape from the phagosomes and multiply in the cytoplasm to become intracellular bacteria, presenting a challenge for antibiotics to reach the bacterial cells and consequently making treatment difficult. In light of this, we employed two cis–trans molecules with aggregation-induced emission (AIE) properties, (E)- and (Z)-TPE-EPy, which have the ability to hinder Gram-positive (G+) bacteria Staphylococcus aureus (S. aureus) and methicillin-resistant S. aureus (MRSA) biofilm formation by reactive oxygen species (ROS) and eradicate intracellular MRSA by host-directed therapy (HDT). These molecules can bind to the intracellular bacteria, target mitochondria, and generate ROS in situ, reduce mitochondrial membrane potential, subsequently induce autophagy to clear intracellular bacteria, and reduce inflammation. Also, these AIE luminogens (AIEgens) can promote the healing of wounds with MRSA infection by killing bacteria and regulating wound inflammation. Our findings shed a light on the potential application of AIEgens in antimicrobial therapy and developed an available strategy against intracellular bacteria.
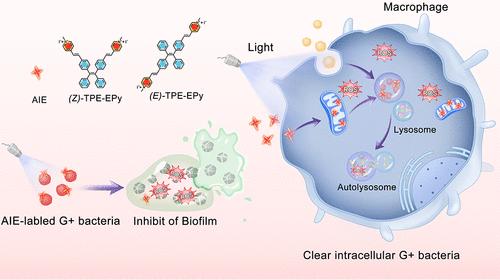
立体异构体AIE光敏剂用于生物膜抑制和宿主定向消除细胞内多重耐药细菌
细菌可以在其表面形成生物膜,也可以从吞噬体中逃脱并在细胞质中繁殖成为细胞内细菌,这对抗生素到达细菌细胞提出了挑战,从而使治疗变得困难。鉴于此,我们采用了两种具有聚集诱导发射(AIE)特性的顺式反式分子(E)-和(Z)- tpe - epy,它们能够通过活性氧(ROS)阻碍革兰氏阳性(G+)细菌金黄色葡萄球菌(S. aureus)和耐甲氧西林金黄色葡萄球菌(MRSA)生物膜的形成,并通过宿主定向治疗(HDT)根除细胞内MRSA。这些分子可以结合胞内细菌,靶向线粒体,原位产生ROS,降低线粒体膜电位,诱导自噬清除胞内细菌,减轻炎症。此外,这些AIE发光原(AIEgens)可以通过杀死细菌和调节伤口炎症来促进MRSA感染伤口的愈合。我们的发现揭示了AIEgens在抗菌治疗中的潜在应用,并开发了一种对抗细胞内细菌的有效策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


