Clustering for methane
IF 44.6
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
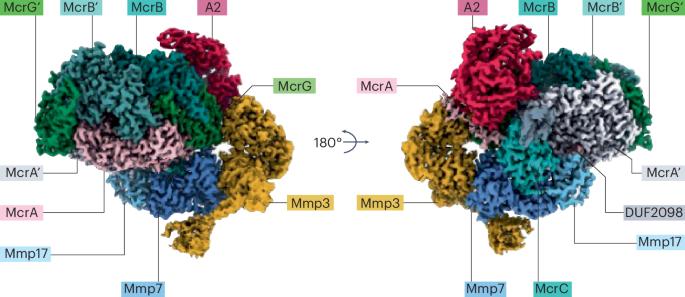
甲烷聚类
研究小组发现,ATP对于MCR甲烷的产生是必需的,并且它也影响MCR激活复合物中亚基的结合。事实上,高分辨率低温电镜结构证实,ATP分子的存在稳定了MCR复合物内的A2亚基。A2成分仅与MCR异源三聚体中的一种不对称结合,而MCR内的锁存样结构调节进入F430空腔。有趣的是,这一特性发挥了作用,允许F430配合物暴露在配合物的近端,因此所含的Ni中心可以还原为Ni(I),并与CoM-SH中的硫醇形成新生的配位。对F430的潜在电子路径进行了进一步的结构和电子研究,从而确定了McrC、Mmp7和Mmp17协调的三种电子密度。它们中的每一个都是由两个由带硫原子桥接的[4Fe-3S]簇组成的,以一种让人联想到氮酶中涉及的m簇前体[8Fe-9S-C]中的l簇拓扑结构。这一特征使研究人员了解了哪个酶家族- MCR或氮酶-首先发展了这种类型的簇:通过系统发育研究,研究小组证实这些簇首先被MCR用于甲烷生成,后来被纳入氮酶酶支架。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


