Capture to convert
IF 44.6
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
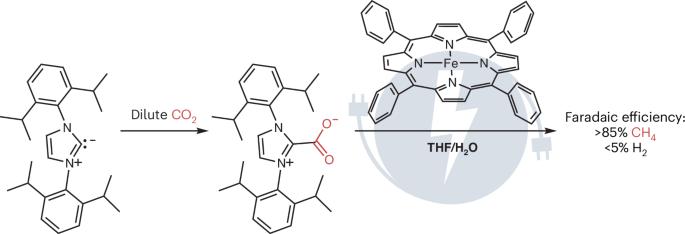
捕获转换
n -杂环碳(1,3-二(2,6-二异丙基苯基)咪唑-2-乙基);由于其高CO2结合和活化能力以及在电催化条件下的抗还原能力而被选中。发现该吸附剂与0.04-10%范围内的稀释CO2源定量反应。在四氢呋喃(THF)溶液中,分子电催化剂四苯基卟啉铁(如图)能够在外加电位下直接还原二氧化碳络合物。值得注意的是,即使以水作为质子源,也观察到对碳产物的高选择性。此外,主要产物是8电子还原的CH4(85%的法拉第效率),尽管这种铁卟啉催化剂在没有二氧化碳的情况下直接还原CO2时通常倾向于2电子还原的CO。该报告支持了集成电化学CO2捕获和转化可能是直接利用稀CO2排放的有效策略的观点。出乎意料的是,它还揭示了吸附剂可以作为催化助剂的作用,以改变给定催化剂对更不寻常产物的固有选择性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


