A polyheteroatomic Diels–Alderase
IF 44.6
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
多杂原子双醛酶
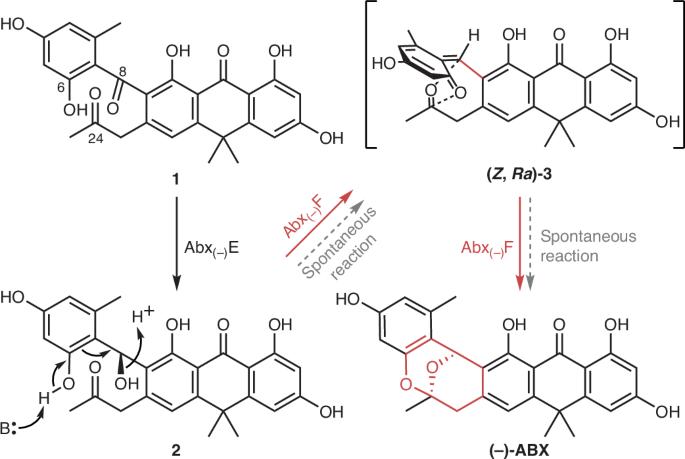
现在,赵一磊、Mehdi Mobli和徐东报道了一种多杂原子Diels-Alderase,它可以催化C=O二烯与C=O二烯的分子内环加成反应。研究人员此前曾致力于阐明聚酮类抗生素(−)- anthrababenzoxocinone(−)-ABX的生物合成。然而,苯二甲基兰王座(1)氧桥三环缩醛(−)-ABX的结构尚不清楚。在目前的工作中,研究继续敲除生物合成基因簇的基因并分析反应产物。结果表明,1被Abx(−)E酶立体特异性还原为化合物2,随后被Abx(−)F酶立体特异性转化为(−)-ABX(如图)。使用纯化酶的体外研究证实了这一点,结果显示Abx(−)F与2的kcat/KM值为3.43±0.23 min−1 μM−1。机理实验都表明Abx(−)F是一种双功能酶,通过将化合物2脱水成反应性的邻醌甲基中间体(Z, Ra)-3和随后的双氧基HDA(如图所示)来催化(−)-ABX的形成。通过核磁共振波谱和求解Abx(−)F的晶体结构,获得了结构上的见解。这使得鉴定活性侧残基,然后突变,以探索反应机制。通过这种方法,我们发现Asp17作为一个一般的碱基,介导脱水生成邻醌甲基中间体(Z, Ra)-3,并且在酶的活性位点上发现了控制随后的双氧基HDA的立体化学的残基。这项工作扩大了生物催化Diels-Alder反应的范围,并显示了酶控制反应中间体和立体选择性的能力。多杂原子diels - alderase为复杂生物活性分子的可持续合成提供了希望,对药物发现具有重要意义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


