Structural basis of GluK2 kainate receptor activation by a partial agonist
IF 10.1
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
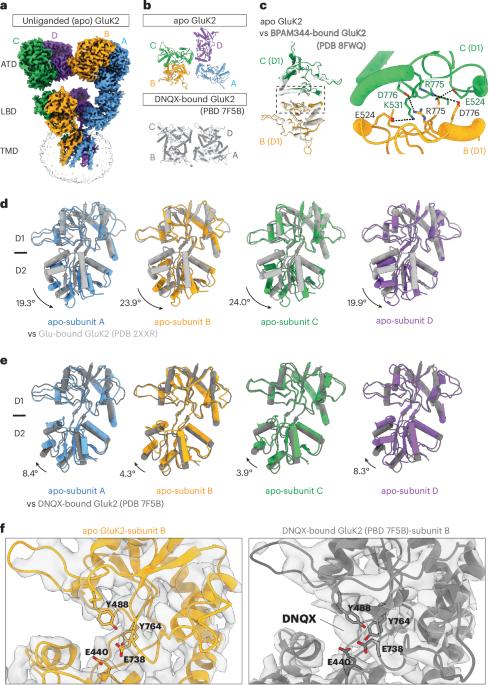
Kainate receptors (KARs) belong to the family of ionotropic glutamate receptors that regulate neurotransmitter release and excitatory synaptic transmission in the central nervous system. Despite their critical roles in synaptic signaling and disease, the detailed gating mechanisms of KARs are not completely understood. Here we present cryo-electron microscopy structures of homomeric rat GluK2 KAR in an unliganded apo state and in complexes with a partial agonist, domoate. Partial agonist-bound GluK2 populates multiple conformations, including intermediate and desensitized states. Moreover, we demonstrate that the N-glycans at the amino-terminal domain–ligand binding domain (LBD) interface modulate receptor gating properties by interfering with cation binding at the LBD dimer interface. Together, these results provide insights into the unique gating mechanisms of KARs. Segura-Covarrubias et al. provide structures of kainate-type ionotropic glutamate receptors in ligand-free and partial agonist-bound conformations. They describe a gating switch upon desensitization and the allosteric modulation of N-glycosylation, which interferes with cation binding.

部分激动剂激活GluK2盐酸盐受体的结构基础
盐酸盐受体(KARs)属于嗜离子性谷氨酸受体家族,在中枢神经系统中调节神经递质释放和兴奋性突触传递。尽管它们在突触信号传导和疾病中起着关键作用,但它们的详细门控机制尚不完全清楚。在这里,我们展示了未配体载子状态和与部分激动剂domoate配合物的同源大鼠GluK2 KAR的低温电镜结构。部分激动剂结合的GluK2填充多种构象,包括中间和脱敏状态。此外,我们还证明了氨基末端结构域-配体结合域(LBD)界面上的n -聚糖通过干扰LBD二聚体界面上的阳离子结合来调节受体的门控特性。总之,这些结果提供了对卡尔斯独特的门控机制的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


