Bioinspired Protein-Mineralized Single-Atom Nanozymes for Tumor-Specific Cascade Therapy via Self-Amplifying Catalytic Synergy
IF 12.1
2区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Single-atom catalysts (SACs) are highly promising in biomedical applications due to their unmatched catalytic activity and atomic-level precision, yet their clinical translation is hindered by limited biocompatibility, instability, and lack of tumor targeting. Here, a universal, bioinspired strategy is proposed to construct flexible, biocompatible SACs by leveraging enzymatic protein scaffolds for mineralization of single-atom platinum (Pt). This protein-mineralized platform enables the fabrication of stable dual-functional nanozymes, exemplified by glucose oxidase-coordinated Pt (GOx-Pt), which simultaneously catalyze glucose oxidation and H₂O₂-to-•OH conversion, generating a self-amplifying cascade for reactive oxygen species (ROS) production. To ensure tumor specificity, the GOx-Pt nanozyme is encapsulated within a pH-responsive zeolitic imidazolate framework (ZIF-8), which remains stable under physiological conditions but disintegrates in mildly acidic tumor environments, enabling localized and selective therapeutic activation. This synergistic design not only enhances antitumor efficacy by inducing oxidative stress and glucose depletion but also minimizes systemic toxicity. The resulting ZIF-8@GOx-Pt system achieves robust catalytic stability, selective cytotoxicity, and significant tumor inhibition (53%) in vivo without discernible side effects. This work pioneers a versatile biomineralization approach for engineering SAC-based nanozymes with dual catalytic and tumor-responsive functions, offering a generalizable strategy for next-generation precision cancer therapeutics.
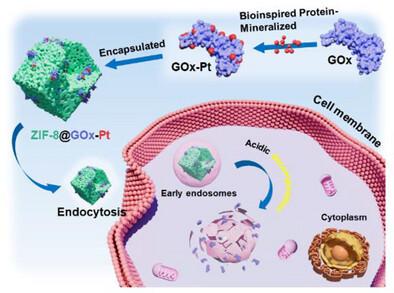
生物启发蛋白矿化单原子纳米酶通过自我放大催化协同作用用于肿瘤特异性级联治疗
单原子催化剂(SACs)由于其无与伦比的催化活性和原子级精度在生物医学应用中具有很高的前景,但其临床转化受到有限的生物相容性,不稳定性和缺乏肿瘤靶向性的阻碍。本文提出了一种通用的、受生物启发的策略,通过利用酶蛋白支架来矿化单原子铂(Pt)来构建灵活的、生物相容性的SACs。这种蛋白质矿化平台能够制造稳定的双功能纳米酶,例如葡萄糖氧化酶协调Pt (GOx-Pt),它同时催化葡萄糖氧化和h2o - O -to- OH转化,产生一个自扩增的级联反应,用于活性氧(ROS)的产生。为了确保肿瘤特异性,GOx-Pt纳米酶被封装在ph响应的沸石咪唑酸框架(ZIF-8)中,该框架在生理条件下保持稳定,但在轻度酸性肿瘤环境中分解,从而实现局部和选择性的治疗激活。这种协同设计不仅可以通过诱导氧化应激和葡萄糖消耗来增强抗肿瘤功效,还可以最大限度地减少全身毒性。由此产生的ZIF-8@GOx-Pt系统在体内具有强大的催化稳定性,选择性细胞毒性和显著的肿瘤抑制(53%),没有明显的副作用。这项工作开创了一种通用的生物矿化方法,用于工程sac纳米酶,具有双重催化和肿瘤反应功能,为下一代精确的癌症治疗提供了一种通用的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Small
工程技术-材料科学:综合
CiteScore
17.70
自引率
3.80%
发文量
1830
审稿时长
2.1 months
期刊介绍:
Small serves as an exceptional platform for both experimental and theoretical studies in fundamental and applied interdisciplinary research at the nano- and microscale. The journal offers a compelling mix of peer-reviewed Research Articles, Reviews, Perspectives, and Comments.
With a remarkable 2022 Journal Impact Factor of 13.3 (Journal Citation Reports from Clarivate Analytics, 2023), Small remains among the top multidisciplinary journals, covering a wide range of topics at the interface of materials science, chemistry, physics, engineering, medicine, and biology.
Small's readership includes biochemists, biologists, biomedical scientists, chemists, engineers, information technologists, materials scientists, physicists, and theoreticians alike.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


