Robust light-harvesting properties upon low-light acclimation in purple bacteria
IF 19.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
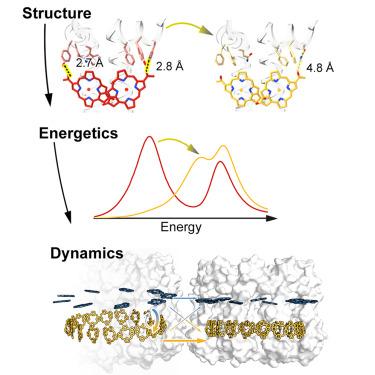
Purple bacteria convert solar energy into biochemical energy with high quantum efficiency across diverse environments. Under low light, many species increase the number of antenna complexes and replace their primary light-harvesting complex 2 (LH2) with a blue-shifted variant, LH3. The structural basis of the blue shift and its influence on the dynamics of solar energy conversion have remained unclear. Here, we integrated cryogenic electron microscopy, ultrafast spectroscopy, and quantum dynamics simulations to compare LH2 and LH3 from Rhodoblastus acidophilus strain 7750. Our analyses revealed that hydrogen bonding dynamically tunes the transition energy, introducing a previously unreported excitation energy equilibrium between bacteriochlorophyll rings in LH3. This energy redistribution opened new inter-complex pathways, enabling 68% faster energy transport to maintain high conversion efficiency even with the larger antenna. Collectively, these results establish structural modifications as a tunable knob to optimize both absorption and transport for robust light harvesting under fluctuating conditions.
紫色细菌在弱光驯化过程中强健的光收集特性
紫色细菌在不同环境下以高量子效率将太阳能转化为生物化学能。在弱光条件下,许多物种增加天线复合物的数量,并用蓝移变体LH3取代其主要的光收集复合物2 (LH2)。蓝移的结构基础及其对太阳能转换动力学的影响尚不清楚。本文采用低温电镜、超快光谱、量子动力学模拟等方法对嗜酸Rhodoblastus acidophilus菌株7750的LH2和LH3进行了比较。我们的分析表明,氢键动态地调节了跃迁能量,在LH3中引入了以前未报道的细菌叶绿素环之间的激发能平衡。这种能量再分配开辟了新的复杂通道,使能量传输速度提高68%,即使使用更大的天线也能保持较高的转换效率。总的来说,这些结果建立了结构修改作为一个可调旋钮,以优化吸收和传输,在波动条件下实现强大的光收集。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chem
Environmental Science-Environmental Chemistry
CiteScore
32.40
自引率
1.30%
发文量
281
期刊介绍:
Chem, affiliated with Cell as its sister journal, serves as a platform for groundbreaking research and illustrates how fundamental inquiries in chemistry and its related fields can contribute to addressing future global challenges. It was established in 2016, and is currently edited by Robert Eagling.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


