Lewis acid-promoted cascade reactions of cyclopropenes: a unified approach to stereoselective synthesis of cyclic ethers and oxaspirolactones†
IF 4.2
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
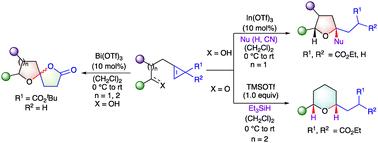
Lewis acid-promoted 5/6-exo trig hydroalkoxylation/reduction cascade of ω-hydroxy cyclopropenes gave expeditious, stereoselective access to THF/THP derivatives. Monoester substituted ω-hydroxy cyclopropenes on treatment with catalytic Bi(OTf)3 lead to [5,5]/[6,5] oxaspirocyclic lactones. This unified strategy relies on generation of transient donor–acceptor (D–A) cyclopropanes from ω-hydroxy cyclopropene precursors. This approach allows for the sequential addition of two nucleophiles in a ‘one pot’ process for the synthesis of THP derivatives. The utility of this methodology is demonstrated in the stereoselective synthesis of a homologue of (±)-civet.
Lewis酸促进环丙烯级联反应:一种立体选择性合成环醚和草螺内酯的统一方法
Lewis酸促进ω-羟基环丙烯的5/6-外三角氢烷氧基化/还原级联反应,Lewis酸促进-羟基环丙烯的5/6-外三角氢烷氧基化/还原级联反应,使THF/THP衍生物具有快速的立体选择性。单酯取代-羟基环丙烯在催化Bi(OTf)3处理下得到[5,5]/[6,5]草螺环内酯。这种统一的策略依赖于从-羟基环丙烯前体生成瞬态供体-受体(D-A)环丙烷。这种方法允许在“一锅”过程中连续添加两种亲核试剂来合成THP衍生物。该方法在(±)-果子狸同系物的立体选择性合成中得到了应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Communications
化学-化学综合
CiteScore
8.60
自引率
4.10%
发文量
2705
审稿时长
1.4 months
期刊介绍:
ChemComm (Chemical Communications) is renowned as the fastest publisher of articles providing information on new avenues of research, drawn from all the world''s major areas of chemical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


