Application-driven design of non-aqueous electrolyte solutions through quantification of interfacial reactions in lithium metal batteries
IF 34.9
1区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
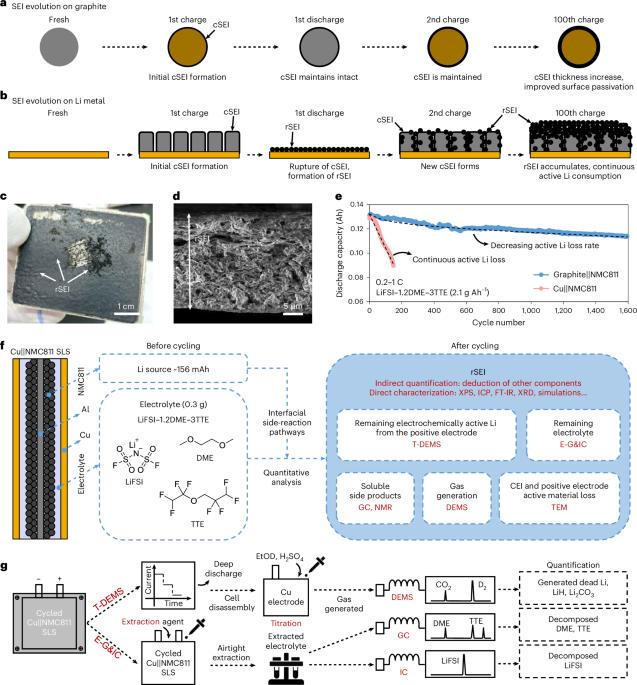
Unwanted side reactions occurring at electrode|electrolyte interfaces significantly impact the cycling life of lithium metal batteries. However, a comprehensive view that rationalizes these interfacial reactions and assesses them both qualitatively and quantitatively is not yet established. Here, by combining multiple analytical techniques, we systematically investigate the interfacial reactions in lithium metal batteries containing ether-based non-aqueous electrolyte solutions. We quantitatively monitor various nanoscale-driven processes such as the reduction and oxidation pathways of lithium salt and organic solvents, the formation of various solid-electrolyte interphase species, the gas generation within the cell and the cross-talk processes between the electrodes. We demonstrate that the consumption of lithium ions owing to the continuous decomposition of the lithium bis(fluorosulfonyl)imide salt, which dominates the interfacial reactions, results in ion depletion during the cell discharge and battery failure. On the basis of these findings, we propose an electrolyte formulation in which lithium bis(fluorosulfonyl)imide content is maximized without compromising dynamic viscosity and bulk ionic conductivity, aiming for long-cycling battery performance. Following this strategy, we assemble and test Li (20 μm thickness)||LiNi0.8Mn0.1Co0.1O2 (17.1 mg cm−2 of active material) single-layer stack pouch cells in lean electrolyte conditions (that is, 2.1 g Ah−1), which can effectively sustain 483 charge (0.2 C or 28 mA)/discharge (1 C or 140 mA) cycles at 25 °C demonstrating a discharge capacity retention of about 77%. Tailored non-aqueous electrolyte solutions are formulated using data obtained from extensive analytical measurements and analyses. These optimized electrolytes improve the cycling performance of single-layer stack lithium metal pouch cells, particularly in lean electrolyte conditions.

应用驱动的非水电解质溶液设计,通过量化锂金属电池中的界面反应
电极与电解液界面发生的不良副反应严重影响锂金属电池的循环寿命。然而,尚未建立一个全面的观点来合理化这些界面反应并定性和定量地评估它们。本文通过多种分析技术的结合,系统地研究了含醚基非水电解质溶液的锂金属电池中的界面反应。我们定量监测各种纳米驱动的过程,如锂盐和有机溶剂的还原和氧化途径,各种固体电解质间相物质的形成,电池内气体的产生以及电极之间的串扰过程。我们证明了锂离子的消耗是由于锂二(氟磺酰基)亚胺盐的持续分解而导致的,而锂二(氟磺酰基)亚胺盐在界面反应中占主导地位,导致电池放电和电池失效期间离子耗尽。基于这些发现,我们提出了一种电解质配方,其中锂二(氟磺酰基)亚胺含量最大化,而不影响动态粘度和体积离子电导率,旨在实现长周期电池性能。根据这一策略,我们组装并测试了Li (20 μm厚度)||LiNi0.8Mn0.1Co0.1O2 (17.1 mg cm−2的活性材料)单层堆叠袋电池,该电池可以在稀薄的电解质条件下(即2.1 g Ah−1)有效地维持483次充电(0.2 C或28 mA)/放电(1 C或140 mA)循环,在25°C下显示放电容量保持率约为77%。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature nanotechnology
工程技术-材料科学:综合
CiteScore
59.70
自引率
0.80%
发文量
196
审稿时长
4-8 weeks
期刊介绍:
Nature Nanotechnology is a prestigious journal that publishes high-quality papers in various areas of nanoscience and nanotechnology. The journal focuses on the design, characterization, and production of structures, devices, and systems that manipulate and control materials at atomic, molecular, and macromolecular scales. It encompasses both bottom-up and top-down approaches, as well as their combinations.
Furthermore, Nature Nanotechnology fosters the exchange of ideas among researchers from diverse disciplines such as chemistry, physics, material science, biomedical research, engineering, and more. It promotes collaboration at the forefront of this multidisciplinary field. The journal covers a wide range of topics, from fundamental research in physics, chemistry, and biology, including computational work and simulations, to the development of innovative devices and technologies for various industrial sectors such as information technology, medicine, manufacturing, high-performance materials, energy, and environmental technologies. It includes coverage of organic, inorganic, and hybrid materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


