Photoelectron Spectroscopy and Ion Chemistry of Benzoxazolide
IF 4.6
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
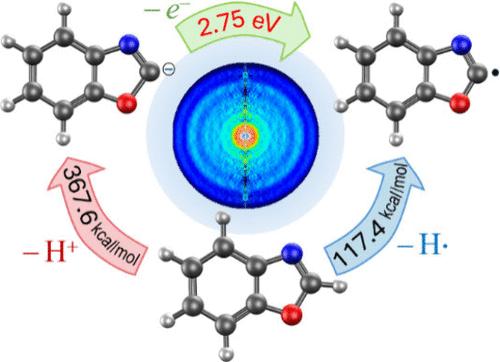
Photoelectron imaging of deprotonated benzoxazole reveals the adiabatic electron affinity of C2-benzoxazolyl σ radical EA0 = 2.75(1) eV. This exceeds the corresponding quantity for oxazolyl by more than 0.5 eV. The extra stabilization of the benzoxazolide anion is attributed to charge sharing between the benzene and oxazole rings. By combining the measured electron affinity of the radical with the calculated acidity of the parent molecule, the C2–H bond dissociation energy of benzoxazole is determined: D0 = 117.4(5) kcal/mol or DH298 = 118.9(5) kcal/mol. In comparison to other closed-shell organic molecules (e.g., benzene), the increased strength of the C2–H bond in benzoxazole indicates a reduced stability of σ-benzoxazolyl due to the impeded delocalization of the radical electron within the heterocyclic part of the molecule. These results have implications for chemical reactivity (e.g., the substitution reactions) of benzoxazole and its derivatives.
苯并恶唑烷的光电子能谱与离子化学
去质子化苯并恶唑的光电子成像表明,c2 -苯并恶唑的σ自由基EA0 = 2.75(1) eV具有绝热电子亲和力。这比恶唑的相应量高出0.5 eV以上。苯并恶唑阴离子的额外稳定性归因于苯和恶唑环之间的电荷共享。结合测量到的自由基的电子亲和力和计算得到的母体分子的酸度,确定苯并恶唑的C2-H键离解能:D0 = 117.4(5) kcal/mol或DH298 = 118.9(5) kcal/mol。与其他闭壳有机分子(如苯)相比,苯并恶唑中C2-H键强度的增加表明,由于分子杂环部分的自由基电子离域受阻,σ-苯并恶唑的稳定性降低。这些结果对苯并恶唑及其衍生物的化学反应性(如取代反应)具有启示意义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry Letters
CHEMISTRY, PHYSICAL-NANOSCIENCE & NANOTECHNOLOGY
CiteScore
9.60
自引率
7.00%
发文量
1519
审稿时长
1.6 months
期刊介绍:
The Journal of Physical Chemistry (JPC) Letters is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, chemical physicists, physicists, material scientists, and engineers. An important criterion for acceptance is that the paper reports a significant scientific advance and/or physical insight such that rapid publication is essential. Two issues of JPC Letters are published each month.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


