Effects of thyroid dysfunctions in the circadian rhythmicity of rat testis
IF 0.9
Q4 GENETICS & HEREDITY
引用次数: 0
Abstract
Background
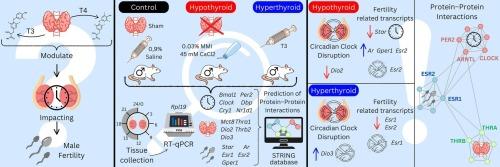
The circadian clock orchestrates the daily rhythmicity of physiological processes in all cells, including the gonadal axis components. Thyroid hormones (THs) regulate both sexes' reproduction homeostasis and steroidogenesis. Thyroid dysfunctions are associated with circadian disruption in a tissue and sex-dependent manner. This study aimed to investigate the effects of thyroid disorders on the rhythmicity of testis circadian clock and its outputs.
Methods
Hypothyroidism and hyperthyroidism were induced in adult male rats and testicular gene expression was assessed every 3 h up to 24 h. Thyroid-stimulating hormone (TSH) and triiodothyronine (T3) serum levels were used to confirm the thyroid states in experimental groups. Rhythmic data were evaluated using One-way ANOVA and 24-h cosine curve data fitting within each group, followed by Two-way ANOVA and pairwise comparisons.
Results
The expression of core clock components (Bmal1, Cry1, and Nr1d1), clock-controlled genes (Dbp, Dio3, and Star) and estrogen receptors (Esr1 and Esr2) exhibited circadian rhythmicity in control testis. Hypothyroidism disrupted the daily oscillations of Bmal1 and Esr2, reduced the mesor of Cry1, Dbp, and Dio3, altered the acrophase of Nr1d1, Cry1 and Esr1 expression, and induced a circadian oscillation on Star expression in testis. Hyperthyroidism disrupted the circadian rhythmicity of Cry1, Dio3 and Esr1 mRNA expression, phase-advanced Bmal1 expression and reduced the mesor of Bmal1 and Esr2 mRNA contents.
Conclusion
Thyroid dysfunction impairs the rhythmicity of the testicular circadian clock and its outputs, as well as the daily expression of genes related to intracellular signaling of thyroid and sexual hormones, which may contribute to the pathogenesis of male reproductive disorders and the impairment of steroidogenesis and spermatogenesis observed under these thyroid dysfunctions.
甲状腺功能障碍对大鼠睾丸昼夜节律的影响
生理时钟协调所有细胞的生理过程的日常节律性,包括性腺轴成分。甲状腺激素(THs)调节两性生殖稳态和甾体生成。甲状腺功能障碍与组织和性别依赖方式的昼夜节律中断有关。本研究旨在探讨甲状腺疾病对睾丸生物钟节律性及其输出的影响。方法诱导成年雄性大鼠甲状腺功能减退和甲状腺功能亢进,每隔3 h ~ 24 h检测睾丸基因表达,采用促甲状腺激素(TSH)和三碘甲状腺原氨酸(T3)检测各组甲状腺状态。每组节律数据采用单因素方差分析和24小时余弦曲线数据拟合进行评估,然后进行双向方差分析和两两比较。结果在对照睾丸中,核心时钟组分(Bmal1、Cry1、Nr1d1)、时钟控制基因(Dbp、Dio3、Star)和雌激素受体(Esr1、Esr2)的表达具有昼夜节律性。甲状腺功能减退症破坏了睾丸Bmal1和Esr2的昼夜振荡,降低了Cry1、Dbp和Dio3的中膜,改变了Nr1d1、Cry1和Esr1的初相表达,并诱导了Star表达的昼夜振荡。甲状腺机能亢进破坏了Cry1、Dio3和Esr1 mRNA表达的昼夜节律性,并降低了Bmal1和Esr2 mRNA的表达量。结论甲状腺功能障碍损害了睾丸生物钟及其输出的节律性,以及甲状腺和性激素细胞内信号相关基因的日常表达,这可能与男性生殖障碍的发病机制以及在甲状腺功能障碍下观察到的类固醇和精子发生的损害有关。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Gene Reports
Biochemistry, Genetics and Molecular Biology-Genetics
CiteScore
3.30
自引率
7.70%
发文量
246
审稿时长
49 days
期刊介绍:
Gene Reports publishes papers that focus on the regulation, expression, function and evolution of genes in all biological contexts, including all prokaryotic and eukaryotic organisms, as well as viruses. Gene Reports strives to be a very diverse journal and topics in all fields will be considered for publication. Although not limited to the following, some general topics include: DNA Organization, Replication & Evolution -Focus on genomic DNA (chromosomal organization, comparative genomics, DNA replication, DNA repair, mobile DNA, mitochondrial DNA, chloroplast DNA). Expression & Function - Focus on functional RNAs (microRNAs, tRNAs, rRNAs, mRNA splicing, alternative polyadenylation) Regulation - Focus on processes that mediate gene-read out (epigenetics, chromatin, histone code, transcription, translation, protein degradation). Cell Signaling - Focus on mechanisms that control information flow into the nucleus to control gene expression (kinase and phosphatase pathways controlled by extra-cellular ligands, Wnt, Notch, TGFbeta/BMPs, FGFs, IGFs etc.) Profiling of gene expression and genetic variation - Focus on high throughput approaches (e.g., DeepSeq, ChIP-Seq, Affymetrix microarrays, proteomics) that define gene regulatory circuitry, molecular pathways and protein/protein networks. Genetics - Focus on development in model organisms (e.g., mouse, frog, fruit fly, worm), human genetic variation, population genetics, as well as agricultural and veterinary genetics. Molecular Pathology & Regenerative Medicine - Focus on the deregulation of molecular processes in human diseases and mechanisms supporting regeneration of tissues through pluripotent or multipotent stem cells.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


