Akt-phosphorylated UFL1 UFMylates ArpC4 to promote metastasis
IF 10.1
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
The role of modification by ubiquitin-fold modifier (‘UFMylation’) in regulating metastasis has remained enigmatic. Cell migration, a critical step in metastasis, is driven by actin polymerization mediated by actin-related proteins 2 and 3 (Arp2/3) at the leading edge of lamellipodia. Here, we report that UFM1-specific E3 ligase 1 (UFL1) interacts with and catalyzes the UFMylation of ArpC4, a core subunit of the Arp2/3 complex. Akt has a key role in this process, which involves phosphorylating UFL1 at T426, thereby enhancing its interaction with ArpC4 and inducing ArpC4 UFMylation. Through ArpC4 UFMylation and potentially other targets, UFL1 facilitates lamellipodia formation and promotes cell migration, invasion and metastasis, making UFL1 an attractive therapeutic target for cancer. Zhao et al. show that Akt-mediated phosphorylation of ubiquitin-fold modifier 1 (UFM1)-specific E3 ligase 1 (UFL1) catalyzes the UFMylation of ArpC4, a core subunit of actin-related proteins 2 and 3. Through ArpC4 UFMylation, UFL1 promotes lamellipodia formation and enhances cell migration, invasion and metastasis.
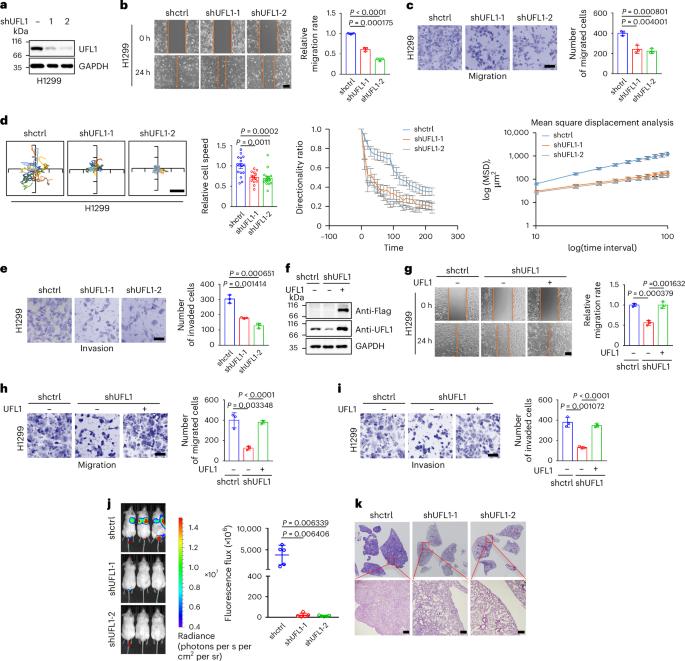

akt磷酸化的UFL1 ufmylate ArpC4促进转移
泛素折叠修饰剂(ufmyation)在调节转移中的作用仍然是谜。细胞迁移是板状足前缘肌动蛋白相关蛋白2和3 (Arp2/3)介导的肌动蛋白聚合驱动的,是转移的关键步骤。在这里,我们报道了ufm1特异性E3连接酶1 (UFL1)与Arp2/3复合物的核心亚基ArpC4相互作用并催化ufmy化。Akt在这一过程中发挥关键作用,通过磷酸化UFL1的T426位点,从而增强其与ArpC4的相互作用,诱导ArpC4 ufmyation。通过ArpC4 UFMylation和潜在的其他靶点,UFL1促进板足形成,促进细胞迁移、侵袭和转移,使UFL1成为一个有吸引力的癌症治疗靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


