TRIP12 structures reveal HECT E3 formation of K29 linkages and branched ubiquitin chains
IF 10.1
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
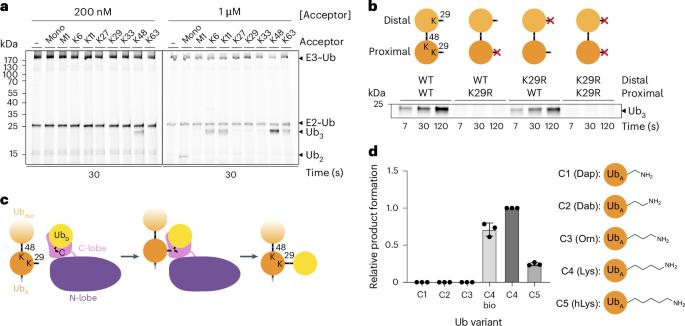
Regulation by ubiquitin depends on E3 ligases forging chains of specific topologies, yet the mechanisms underlying the generation of atypical linkages remain largely elusive. Here we utilize biochemistry, chemistry, and cryo-EM to define the catalytic architecture producing K29 linkages and K29/K48 branches for the human HECT E3 TRIP12. TRIP12 resembles a pincer. One pincer side comprises tandem ubiquitin-binding domains, engaging the proximal ubiquitin to direct its K29 towards the ubiquitylation active site, and selectively capturing a distal ubiquitin from a K48-linked chain. The opposite pincer side—the HECT domain—precisely juxtaposes the ubiquitins to be joined, further ensuring K29 linkage specificity. Comparison to the prior structure visualizing K48-linked chain formation by UBR5 reveals a similar mechanism shared by two human HECT enzymes: parallel features of the E3s, donor and acceptor ubiquitins configure the active site around the targeted lysine, with E3-specific domains buttressing the acceptor for linkage-specific polyubiquitylation. Using biochemistry, chemical biology, and cryo-EM, Maiwald et al. elucidate how TRIP12 forms K29 linkages and K29/K48-linked branched ubiquitin chains, revealing a mechanism for polyubiquitylation shared by some HECT E3s.

TRIP12结构揭示了K29键和分支泛素链的HECT E3形成
泛素的调控依赖于形成特定拓扑链的E3连接酶,然而产生非典型连接的机制在很大程度上仍然难以捉摸。在这里,我们利用生物化学、化学和低温电镜来定义人类HECT E3 TRIP12产生K29键和K29/K48分支的催化结构。TRIP12就像一个钳子。一个钳子侧包含串联泛素结合域,参与近端泛素引导其K29走向泛素化活性位点,并选择性地从k48连接链中捕获远端泛素。相反的钳子侧——HECT结构域——精确地并置待连接的泛素,进一步确保了K29连锁的特异性。与之前的结构相比,通过UBR5可视化k48连接链的形成揭示了两种人类HECT酶共享的类似机制:E3s的平行特征,供体和受体泛素在靶向赖氨酸周围配置活性位点,e3特异性结构域支持受体进行连接特异性多泛素化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


