Astrocytic Inducible Nitric Oxide Synthase Upregulation Contributes to Chronic Below-Level Neuropathic Pain Following Spinal Cord Injury in Male Rats
Abstract
Background
Spinal cord injury (SCI) leads to persistent inflammation, contributing to chronic neuropathic pain. However, current treatments show limited efficacy. Three types of nitric oxide synthase (NOS) play different roles in inflammation and neuronal hyperexcitation. Therefore, this study aimed to determine the predominant NOS subtype involved in neuropathic pain after spinal contusion.
Methods
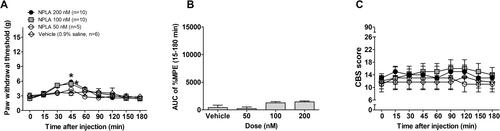
We investigated the effects of intrathecal NOS inhibitors on mechanical sensitivity following a moderate spinal contusion injury in male Sprague-Dawley rats. These NOS inhibitors were N(G)-nitro-L-arginine methyl ester hydrochloride (L-NAME; non-selective NOS inhibitor), 1400W (iNOS inhibitor), Nω-propyl-L-arginine hydrochloride (NPLA; nNOS inhibitor) and N5-(1-iminoethyl)-L-ornithine (L-NIO; eNOS inhibitor). Additionally, we analysed protein expression and cellular localisation of spinal NOS subtypes in rats that underwent SCI or sham procedures.
Results
Treatment with L-NAME significantly reduced paw withdrawal threshold in a dose-dependent manner, although motor deficits appeared at the highest dose (30 μM), while 1400W effectively alleviated mechanical hypersensitivity without motor side effects. NPLA showed limited efficacy, and L-NIO had no effect. Protein expression of iNOS increased two-fold in the L4-5 spinal segment of SCI rats compared with sham controls. After SCI, iNOS-immunoreactivity colocalized with GFAP in the superficial laminae of the L4-5 spinal segment. Treatment with 1400W reduced the hyper-reactivity of both iNOS and GFAP.
Conclusions
These findings indicate that iNOS plays a significant role in below-level neuropathic pain following thoracic spinal cord contusion in rats. Specific blockade of iNOS activity may have potential as a therapeutic intervention for spinal-contusion-induced neuropathic pain with reduced risk of side effects.
Significance Statement
iNOS inhibition effectively alleviated pain without motor side effects, unlike non-selective NOS, nNOS and eNOS inhibitors. The colocalization of iNOS with astrocytes in the spinal cord suggests a key mechanism in pain maintenance. These findings highlight the potential of targeting iNOS as a therapeutic strategy for SCI-induced neuropathic pain with reduced risks of side effects.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


