Aspidosperma-type bisindole alkaloids from Melodinus suaveolens and their antiproliferative activities
IF 3.4
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Melosuadimins A–L (1–12), twelve undescribed monoterpenoid dimeric alkaloids with different subsets of aspidosperma-type alkaloid units were isolated from Melodinus suaveolens. Their structures were elucidated through extensive spectroscopic data analysis. Notably, melosuadimin A (1) was a vindoline-aspidosperma type bisindole alkaloid with a dihydrofuran ring linkage. Melosuadimins B (2) and C (3) were proposed as oxidation and ring-opening derivatives of melosuadimin A. Additionally, melosuadimin D (4), featuring a unique 1,2-oxazinane ring, was possibly produced through rearrangement from melosuadimin B. The in vitro cytotoxic potential of these compounds against three colorectal cancer cell lines was evaluated, revealing that 2 exhibited marked antiproliferative activity, inducing apoptosis and G2/M cell cycle arrest.
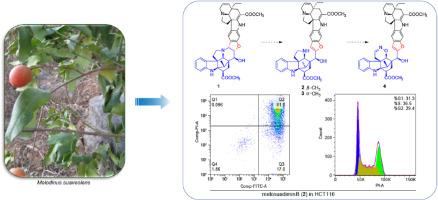
甜麦冬中spispiertype双吲哚类生物碱及其抗增殖活性
melosudimins A-L(1-12)是12种未被描述的单萜类二聚生物碱,具有不同的蛇皮草型生物碱单位亚群。通过广泛的光谱数据分析阐明了它们的结构。值得注意的是,melosuadimin A(1)是一种具有二氢呋喃环连接的vindoline-aspidosperma型双吲哚生物碱。melosuadimin B(2)和C(3)被认为是melosuadimin a的氧化和开环衍生物。此外,melosuadimin D(4)具有独特的1,2-恶嗪环,可能是由melosuadimin B通过重排产生的。这些化合物对三种结直肠癌细胞系的体外细胞毒潜力进行了评估,发现2具有显著的抗增殖活性,诱导细胞凋亡和G2/M细胞周期阻滞。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Phytochemistry
生物-植物科学
CiteScore
6.40
自引率
7.90%
发文量
443
审稿时长
39 days
期刊介绍:
Phytochemistry is a leading international journal publishing studies of plant chemistry, biochemistry, molecular biology and genetics, structure and bioactivities of phytochemicals, including ''-omics'' and bioinformatics/computational biology approaches. Phytochemistry is a primary source for papers dealing with phytochemicals, especially reports concerning their biosynthesis, regulation, and biological properties both in planta and as bioactive principles. Articles are published online as soon as possible as Articles-in-Press and in 12 volumes per year. Occasional topic-focussed special issues are published composed of papers from invited authors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


