Multiple steps of dynein activation by Lis1 visualized by cryo-EM
IF 10.1
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Cytoplasmic dynein-1 (dynein) is an essential molecular motor controlled in part by autoinhibition. Lis1, a key dynein regulator mutated in the neurodevelopmental disease lissencephaly, plays a role in dynein activation. We recently identified a structure of partially autoinhibited dynein bound to Lis1, which suggests an intermediate state in dynein’s activation pathway. However, other structural information is needed to fully understand how Lis1 activates dynein. Here, we used cryo-EM and yeast dynein and Lis1 incubated with ATP at different time points to reveal conformations that we propose represent additional intermediate states in dynein’s activation pathway. We solved 16 high-resolution structures, including 7 distinct dynein and dynein–Lis1 structures from the same sample. Our data support a model in which Lis1 relieves dynein autoinhibition by increasing its basal ATP hydrolysis rate and promoting conformations compatible with complex assembly and motility. Together, this analysis advances our understanding of dynein activation and the contribution of Lis1 to this process. Using cryo-EM, Kendrick et al. reveal multiple dynein conformations during dynein’s mechanochemical cycle and Lis1 binding that represent intermediate states of dynein’s activation.
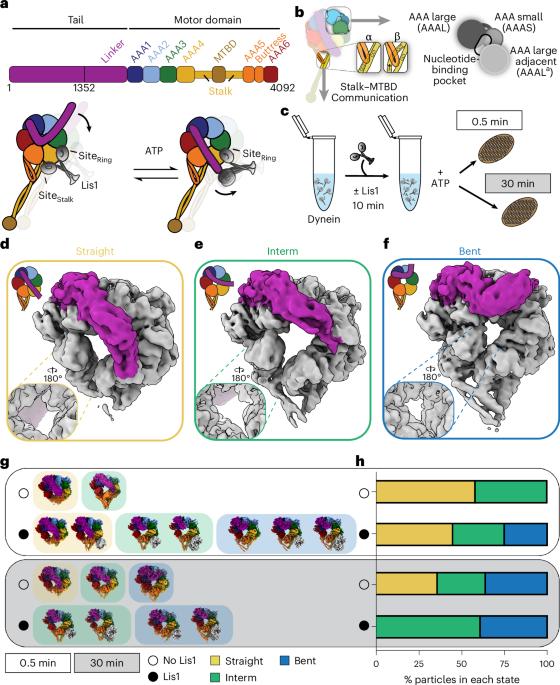

低温电镜观察Lis1激活动力蛋白的多个步骤
胞质动力蛋白-1 (dynein)是一种重要的分子马达,部分受自抑制控制。Lis1是神经发育疾病无脑畸形中发生突变的关键动力蛋白调节因子,在动力蛋白激活中起作用。我们最近发现了一个与Lis1结合的部分自抑制动力蛋白结构,这表明动力蛋白的激活途径处于中间状态。然而,为了充分了解Lis1如何激活动力蛋白,还需要其他的结构信息。在这里,我们使用低温电镜和酵母动力蛋白和Lis1在不同时间点与ATP孵育,以揭示我们提出的代表动力蛋白激活途径中额外中间状态的构象。我们解决了16个高分辨率结构,包括来自同一样品的7个不同的dynein和dynein - lis1结构。我们的数据支持一个模型,即Lis1通过增加其基础ATP水解率和促进与复杂组装和运动相容的构象来减轻动力蛋白的自抑制。总之,这一分析促进了我们对动力蛋白激活和Lis1对这一过程的贡献的理解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


