Rate-limiting regimes in photochemical H2 generation by complexes of colloidal CdS nanorods and hydrogenase
IF 19.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Driving redox enzyme catalysis with photoexcited semiconductor nanocrystals is a compelling approach for chemical conversion. We examined how the interplay of the many chemical steps involved determines the rates of photochemical H2 production with complexes of colloidal CdS nanorods and an [FeFe]-hydrogenase. We elucidated the roles of three critical and previously elusive processes—scavenging of photoexcited holes from nanorods, back-electron transfer, and H2 oxidation. Kinetic Monte Carlo simulations and fitting to experimental data revealed that hole transfer becomes the rate-limiting step at high illumination intensities. Comparisons of simulations to experimental H2 production showed that both back-electron transfer and H2 oxidation play an efficiency-limiting role at high catalyst loadings. This work provides guiding principles for tuning experimental parameters to minimize energy-wasting pathways and optimize photochemical product formation. More broadly, we demonstrate how critical but elusive chemical steps in photochemical reactions can be probed with a combination of experiments and simulations.
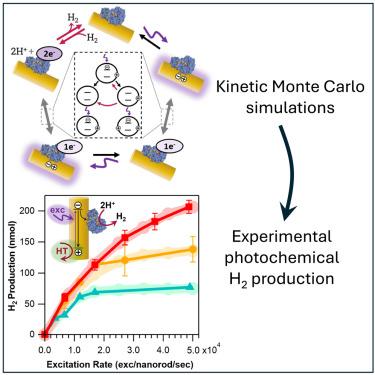
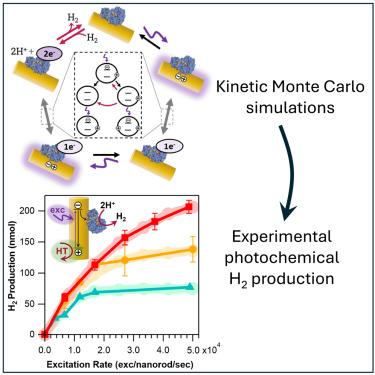
胶体CdS纳米棒与氢化酶配合物光化学制氢的限速机制
光激发半导体纳米晶体驱动氧化还原酶催化是化学转化的一种引人注目的方法。我们研究了所涉及的许多化学步骤的相互作用如何决定胶体CdS纳米棒和[FeFe]-氢化酶复合物光化学制氢的速率。我们阐明了三个关键的和以前难以捉摸的过程-从纳米棒中清除光激发空穴,反向电子转移和H2氧化。动力学蒙特卡罗模拟和实验数据拟合表明,在高光照强度下,空穴转移成为速率限制步骤。与实验制氢的模拟比较表明,在高催化剂负载下,后电子转移和H2氧化都起着限制效率的作用。这项工作为调整实验参数以减少能量浪费途径和优化光化学产物形成提供了指导原则。更广泛地说,我们展示了如何通过实验和模拟的结合来探索光化学反应中关键但难以捉摸的化学步骤。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chem
Environmental Science-Environmental Chemistry
CiteScore
32.40
自引率
1.30%
发文量
281
期刊介绍:
Chem, affiliated with Cell as its sister journal, serves as a platform for groundbreaking research and illustrates how fundamental inquiries in chemistry and its related fields can contribute to addressing future global challenges. It was established in 2016, and is currently edited by Robert Eagling.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


