Tuning Hydrogen Adsorption in B4CN3 Monolayers: The Role of Metal Decoration and Vacancy Defects
IF 8.2
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
This study investigates the hydrogen storage performance of various metal-decorated pristine and defective B4CN3 monolayers using first-principles calculations. The selected metals span alkali, alkaline earth, and 3d transition-metal (TM) series. All metal-decorated B4CN3 systems exhibit thermodynamic stability, as illustrated through their negative binding energies. The adsorption behavior and interaction strength of hydrogen are influenced by the type of metal, with alkali and alkaline earth metals showing weak physisorption and TMs demonstrating moderate to strong interactions via Kubas adsorption modes. The adsorption strength between metal atoms and hydrogen is crucial in determining the efficiency of hydrogen storage materials. In particular, Li-decorated pristine B4CN3 achieves a maximum gravimetric hydrogen storage capacity of 12.59 wt %, but its desorption temperature is too low due to the weak physisorption. To improve the hydrogen storage properties, vacancy defects were introduced. Among the investigated vacancy defects, the carbon vacancy (VC) is the most energetically favorable. VC leads to a stronger hydrogen adsorption energy and higher desorption temperature. This improvement is attributed toa shift in the Fermi level toward the vacuum level, which increases the polarizability of the substrates and enhances the H2 adsorption. In addition, practical hydrogen storage assessed using ab initio molecular dynamic simulations at various desorption temperatures and pressures reveals that Mg, Ca, and Sc are promising candidates for pristine B4CN3, while Li, Na, K, and Ca were identified for defective B4CN3. This work provides valuable insights for the development of advanced hydrogen storage systems that leverage defective B4CN3 monolayers.
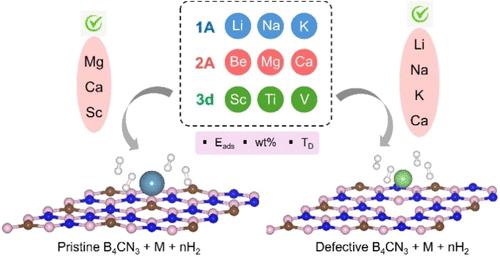
B4CN3单分子膜的氢吸附:金属修饰和空位缺陷的作用
本研究利用第一性原理计算研究了各种金属修饰的原始和缺陷B4CN3单层的储氢性能。所选金属涵盖碱金属、碱土和3d过渡金属(TM)系列。所有金属修饰的B4CN3体系都表现出热力学稳定性,如它们的负结合能所示。氢的吸附行为和相互作用强度受金属类型的影响,碱金属和碱土金属通过Kubas吸附模式表现为弱物理吸附,而TMs通过Kubas吸附模式表现为中强相互作用。金属原子与氢之间的吸附强度是决定储氢材料效率的关键。特别是,锂修饰的原始B4CN3的最大重量储氢容量为12.59 wt %,但由于物理吸附弱,其解吸温度过低。为了提高储氢性能,引入了空位缺陷。在所研究的空位缺陷中,碳空位(VC)是最有利的。VC对氢的吸附能更强,解吸温度更高。这种改进归因于费米能级向真空能级的移动,这增加了衬底的极化率,增强了H2的吸附。此外,在不同的解吸温度和压力下,通过从头算分子动力学模拟评估了实际的氢储存,结果表明Mg、Ca和Sc是原始B4CN3的有希望的候选物,而Li、Na、K和Ca被确定为缺陷B4CN3的候选物。这项工作为开发利用有缺陷的B4CN3单层的先进储氢系统提供了有价值的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


