Kinetic vs Thermodynamic Controlled Promiscuous Biocatalytic Asymmetric Henry Reaction in Diastereocomplementary Synthesis of β-Nitroalcohols
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Exploring promiscuous catalytic activity of enzymes to stereoselective transformations uncovers ecofriendly asymmetric catalysts and provides scope to execute new-to-nature chemistry. Biocatalytic promiscuous diastereoselective Henry reaction (DHR) is restricted to a few hydroxynitrile lyases (HNLs), often engineered, with narrow synthetic scope and anti-selectivity. Here, we report a single native enzyme exhibiting complementary diastereoselectivity in an asymmetric Henry reaction. Baliospermum montanum HNL (BmHNL)-catalyzed DHR enabled the production of thirty-two anti-(1S,2R)-β-nitroalcohols with up to >99% conversion, >99% ee, and >99% de using longer nitroalkanes. It afforded syn diastereomers, i.e., (1S,2S)-β-nitroalcohols as thermodynamically controlled products by manipulating the reaction conditions. Reuse of BmHNL in DHR for 20 cycles has empowered the synthesis of (1S,2R)-2-nitro-1-phenylpropan-1-ol (NPP), where it retained 82% of its initial activity, maintained >99% ee on each cycle, and provided total turnover number >3.7 × 104, which is 140-fold higher compared to the only existing biocatalytic DHR. Our study demonstrates a gram-scale synthesis of (1S,2R)-NPP and a preparative-scale synthesis of (1S,2S)-NPP, a precursor to d-norpseudoephedrine used for the treatment of obesity. The origin of the inverse diastereoselectivity was probed using isotope labeling studies. Molecular modeling of the reaction using density functional theory methods reveals the underlying mechanism of the diastereoselectivity and the competing kinetic vs thermodynamic nature of the reaction. Despite a promiscuous catalytic activity, the outstanding catalytic efficiency, broad synthetic scope, and complementary diastereoselectivity of the native enzyme of BmHNL on DHR are remarkable, which illustrates it as a specialized biocatalyst for greener technology and stereocontrolled production of diverse β-nitroalcohols.
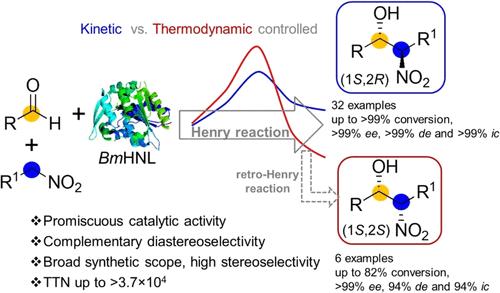
非对映互补合成β-硝基醇的动力学与热力学控制混杂生物催化不对称Henry反应
探索酶对立体选择转化的混杂催化活性揭示了生态友好的不对称催化剂,并提供了执行新自然化学的范围。生物催化混杂非对选择性亨利反应(DHR)仅限于几种羟基腈裂解酶(hnl),通常是工程化的,合成范围窄,具有抗选择性。在这里,我们报道了一种在不对称Henry反应中表现出互补非对映选择性的单一天然酶。Baliospermum monmonum HNL (BmHNL)催化的DHR使32种抗(1S,2R)-β-硝基醇的转化率高达>;99%, ee >99%, de >99%。通过控制反应条件,可以得到(1S,2S)-β-硝基醇等对映异构体作为热力学控制产物。BmHNL在DHR中重复使用20个循环,使(1S,2R)-2-硝基-1-苯基丙烷-1-醇(NPP)的合成保持了82%的初始活性,在每个循环中保持了99%的ee,并提供了3.7 × 104的总周转率,比现有的唯一生物催化DHR高140倍。我们的研究证明了(1S,2R)-NPP的克级合成和(1S,2S)-NPP的制备级合成,(1S,2S)-NPP是用于治疗肥胖的d-去甲麻黄碱的前体。利用同位素标记研究探讨了逆非对映选择性的起源。利用密度泛函理论方法对反应进行分子模拟,揭示了反应非对映选择性的潜在机理以及反应的动力学和热力学性质。尽管具有混杂的催化活性,但BmHNL的天然酶在DHR上具有出色的催化效率、广泛的合成范围和互补的非对映选择性,这表明它是一种专门的生物催化剂,可用于绿色技术和立体控制生产多种β-硝基醇。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


