A Theoretical and Experimental Study of Carbonized Lignin from Alfa Fibers as a Clay-Based Adsorbent
IF 3.9
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
In the current work, a novel carbonaceous composite was successfully synthesized via pyrolysis utilizing lignin extracted from Raw Stipa Tenacissima fibers, locally referred to as Alfa fibers, combined with clay. This composite material was developed with the aim of enhancing the removal efficiency of anionic dyes from wastewater, particularly methyl orange (MO), which is commonly used in the textile industry and known for its persistence in aquatic environments. A comprehensive physicochemical characterization of the adsorbent was conducted to know its surface and structural properties. Zeta potential analysis provided insight into the surface charge behavior, The Brunauer–Emmett–Teller method revealed a significant increase in specific surface area postcarbonization, which correlates with improved adsorption performance. The successful incorporation of carbon on the clay surface was further confirmed by EDX mapping, indicating a uniform and stable distribution of carbonaceous matter throughout the composite matrix. The adsorption performance of the material was evaluated through batch adsorption experiments. Key operational parameters such as adsorbent dosage, contact time, initial dye concentration, temperature, and pH were optimized to identify the conditions leading to maximum removal efficiency. Kinetic modeling indicated that the adsorption behavior closely followed the pseudo-first-order model, suggesting that the process is governed by physisorption mechanisms. A remarkable improvement in adsorption capacity was observed upon carbonization, with the composite exhibiting a maximum equilibrium adsorption capacity (Qe) of 228.58 mg/g, compared to 110.07 mg/g for pristine clay. To gain molecular-level insights into the adsorption mechanism, density functional theory calculations and Monte Carlo simulations were employed. These theoretical investigations revealed stronger binding affinities of MO molecules on the composite surface, primarily driven by electrostatic interactions and hydrogen bonding between the dye molecules and the surface functional groups introduced during carbonization.
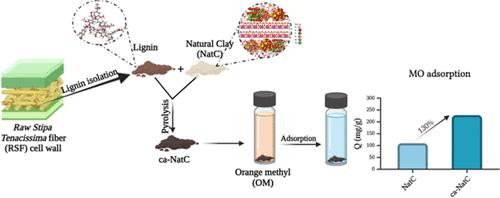
α纤维炭化木质素作为粘土基吸附剂的理论与实验研究
在目前的工作中,利用从原刺茅纤维(当地称为阿尔法纤维)中提取的木质素与粘土结合,通过热解成功合成了一种新型的碳质复合材料。开发这种复合材料的目的是提高废水中阴离子染料的去除效率,特别是甲基橙(MO),甲基橙通常用于纺织工业,并以其在水生环境中的持久性而闻名。对吸附剂进行了全面的物理化学表征,以了解其表面和结构性质。Zeta电位分析提供了对表面电荷行为的深入了解,brunauer - emmet - teller方法揭示了碳化后比表面积的显著增加,这与改善的吸附性能有关。EDX图进一步证实了碳在粘土表面的成功结合,表明碳质物质在整个复合基质中的分布均匀而稳定。通过批量吸附实验对该材料的吸附性能进行了评价。对吸附剂用量、接触时间、初始染料浓度、温度和pH等关键操作参数进行了优化,以确定最大去除效率的条件。动力学模拟表明,吸附行为符合拟一阶模型,表明吸附过程受物理吸附机制控制。碳化后,复合材料的最大平衡吸附容量(Qe)为228.58 mg/g,而原始粘土的最大平衡吸附容量为110.07 mg/g。为了在分子水平上深入了解吸附机理,采用了密度泛函理论计算和蒙特卡罗模拟。这些理论研究表明MO分子在复合材料表面的结合亲和力更强,这主要是由静电相互作用和炭化过程中引入的染料分子与表面官能团之间的氢键驱动的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


