A randomised, double-blind, placebo-controlled evaluation of polyphenol-based standardised cinnamon bark extract in patients with mild-to-moderate COVID-19
IF 1.9
4区 医学
Q2 INTEGRATIVE & COMPLEMENTARY MEDICINE
引用次数: 0
Abstract
Objectives
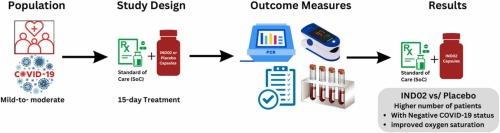
To evaluate the efficacy and safety of oral supplementation of polyphenol-based standardised cinnamon bark extract (IND02) capsules to ‘standard of care’ (SoC) in mild-to-moderate patients with coronavirus disease (COVID-19) in a randomised double-blind, placebo-controlled study.
Design and methods
A total of 118 participants (aged 18–60 years, both sexes) were randomised to have 15 days of regimen of IND02 or placebo capsules in two stages: a loading dose of 3 g/d for the initial three days and a maintenance dose of 1.5 g/d for the subsequent 12 days. Efficacy and safety outcome measures were based on guidelines issued by ‘Ministry of Health and Family Welfare, Government of India’.
Results
All patients in both treatment groups showed clinical recovery and improvement without significant differences between the groups. The number of patients with negative COVID-19 status and having enhanced (>2% increase) oxygen saturation (SpO2) was significantly (P = 0.039 and P = 0.049, respectively) higher in the IND02 supplemented group (vs. Placebo), but no significant differences in other outcome measures between the groups. No serious adverse events, deaths, or treatment-related adverse effects were observed. None of the patients in either group required discontinuation of treatment or ventilation support, progressed to a severe stage, or had changes in vital signs or laboratory examination results.
Conclusion
IND02 supplementation with SoC in COVID-19 patients with mild-to-moderate symptoms was safe and well-tolerated, with improved oxygen saturation and an increased number of patients achieving negative status for clinical recovery and improvement.
一项随机、双盲、安慰剂对照的基于多酚的标准化肉桂皮提取物对轻中度COVID-19患者的评估
目的:在一项随机双盲、安慰剂对照研究中,评估口服多酚基标准肉桂皮提取物(IND02)胶囊对轻中度冠状病毒病(COVID-19)患者的“标准护理”(SoC)的疗效和安全性。设计和方法总共118名参与者(18-60岁,男女)被随机分为两个阶段,接受15天的IND02或安慰剂胶囊方案:最初三天的负荷剂量为3g /d,随后12天的维持剂量为1.5 g/d。有效性和安全性结果措施是根据“印度政府卫生和家庭福利部”发布的准则制定的。结果两组患者临床症状均有所恢复和改善,两组间差异无统计学意义。与安慰剂组相比,IND02补充组的COVID-19阴性和血氧饱和度(SpO2)升高(增加2%)的患者人数显著(P = 0.039和P = 0.049)增加,但两组之间的其他结局指标无显著差异。未观察到严重的不良事件、死亡或治疗相关的不良反应。两组均无患者需要停止治疗或通气支持,进展到严重阶段,或有生命体征或实验室检查结果的变化。结论ind02加SoC治疗轻至中度症状的COVID-19患者安全且耐受性良好,血氧饱和度改善,阴性状态患者数量增加,临床恢复和改善。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Herbal Medicine
INTEGRATIVE & COMPLEMENTARY MEDICINE-
CiteScore
3.90
自引率
0.00%
发文量
94
期刊介绍:
The Journal of Herbal Medicine, the official journal of the National Institute of Medical Herbalists, is a peer reviewed journal which aims to serve its readers as an authoritative resource on the profession and practice of herbal medicine. The content areas of the journal reflect the interests of Medical Herbalists and other health professionals interested in the clinical and professional application of botanical medicines. The objective is to strengthen the research and educational base of herbal medicine with research papers in the form of case studies, original research articles and reviews, monographs, clinical trials and relevant in vitro studies. It also publishes policy statements, opinion pieces, book reviews, conference proceedings and profession related information such as pharmacovigilance reports providing an information source for not only the Herbal Practitioner but any Health professional with an interest in phytotherapy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


