Low-Temperature Imidized Polyimide Binder Enabling Structural and Electrochemical Stability of NCM811 Cathode for High-Safety and Long-Cycling Lithium-Ion Batteries
IF 7.3
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
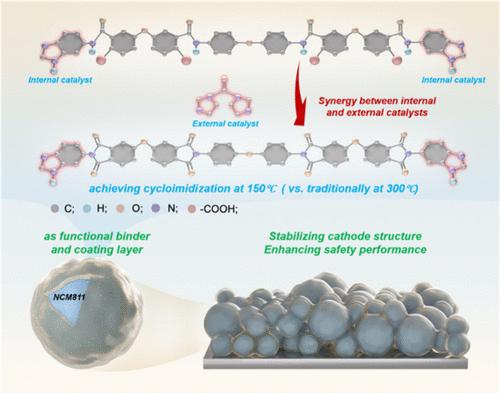
Polyimide (PI) is a promising binder material for lithium-ion batteries due to its excellent mechanical strength, high thermal stability, and outstanding chemical inertness. However, the high-temperature imidization requirement (>300 °C) of traditional PI binders is incompatible with current electrode preparation processes, severely limiting their practical applications. In this study, a low-temperature-imidized polyimide (∼150 °C) was elaborately designed and utilized as NCM811 cathode binder, which works by covalently bonding 5-aminobenzimidazole (ABZ) to the ends of the flexible 4,4′-oxidiphthalic anhydride (ODPA)/4,4′-oxidiphenylamine (ODA) poly(amic acid) (PAA) chains to realize the self-catalysis at a low temperature, followed by the assistance of dual-functional N,N′-carbonyldiimidazole (CDI) as a catalyst and dehydrating agent to accelerate the condensation and low-temperature dehydration of amic acid, yielding the imidization of the PAA precursor to give PI at 150 °C. Compared to the conventional PVDF binder, the cathode utilizing this low-temperature imidized polyimide (LTPI) binder exhibited excellent capacity retention (84.9% vs 81.7%) after 100 cycles at 0.2C, and superior rate performance (119.3 mAh·g–1 vs 95.7 mAh·g–1 at 5.0C) under a 4.3 V cutoff voltage. The CV, SEM, XRD, and XPS results demonstrate that the designed LTPI binder effectively suppresses the hexagonal (H2) to hexagonal (H3) phase transition and transition metal dissolution, maintaining the cathode’s structural integrity during charge–discharge cycles, thereby ensuring excellent long-term cycle stability. Furthermore, the NCM811 cathode with LTPI binder exhibits significantly enhanced flame-retardant performance compared with the NCM811/PVDF cathode in the combustion experiments, demonstrating the superiority of LTPI binders in improving the safety of the cathode materials. The NCM811/LTPI cathode retains the excellent structural and electrochemical stability of traditional high-temperature-imidized PI binders, providing a practical pathway to develop high-performance binder materials for high-safety and long-cycling lithium-ion batteries.
低温亚胺化聚酰亚胺粘结剂对NCM811高安全和长循环锂离子电池阴极结构和电化学稳定性的影响
聚酰亚胺(PI)具有优异的机械强度、高的热稳定性和优异的化学惰性,是一种很有前途的锂离子电池粘结材料。然而,传统PI粘结剂的高温亚胺化要求(>300°C)与目前的电极制备工艺不兼容,严重限制了其实际应用。在本研究中,精心设计了一种低温亚酰化聚酰亚胺(~ 150℃)作为NCM811阴极粘结剂,通过将5-氨基苯并咪唑(ABZ)共价结合到柔性的4,4 ' -氧化邻苯酐(ODPA)/4,4 ' -氧化苯胺(ODA)聚胺酸(PAA)链的末端,实现低温自催化。然后用双官能团N,N ' -羰基二咪唑(CDI)作为催化剂和脱水剂,加速酰胺的缩合和低温脱水,在150℃下得到PAA前驱体亚酰化得到PI。与传统的PVDF粘合剂相比,使用这种低温亚酰化聚酰亚胺(LTPI)粘合剂的阴极在0.2C下循环100次后表现出优异的容量保持率(84.9% vs 81.7%),在4.3 V截止电压下表现出优异的倍率性能(119.3 mAh·g-1 vs 95.7 mAh·g-1)。CV、SEM、XRD和XPS结果表明,所设计的LTPI粘结剂有效抑制了六方(H2)到六方(H3)的相变和过渡金属的溶解,保持了阴极在充放电循环过程中的结构完整性,从而保证了优异的长期循环稳定性。此外,与NCM811/PVDF阴极相比,LTPI粘结剂的NCM811阴极在燃烧实验中表现出显著的阻燃性能,证明了LTPI粘结剂在提高阴极材料安全性方面的优势。NCM811/LTPI阴极保留了传统高温亚甲基化PI粘结剂的优良结构和电化学稳定性,为开发高安全性、长循环锂离子电池的高性能粘结剂材料提供了一条实用途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Sustainable Chemistry & Engineering
CHEMISTRY, MULTIDISCIPLINARY-ENGINEERING, CHEMICAL
CiteScore
13.80
自引率
4.80%
发文量
1470
审稿时长
1.7 months
期刊介绍:
ACS Sustainable Chemistry & Engineering is a prestigious weekly peer-reviewed scientific journal published by the American Chemical Society. Dedicated to advancing the principles of green chemistry and green engineering, it covers a wide array of research topics including green chemistry, green engineering, biomass, alternative energy, and life cycle assessment.
The journal welcomes submissions in various formats, including Letters, Articles, Features, and Perspectives (Reviews), that address the challenges of sustainability in the chemical enterprise and contribute to the advancement of sustainable practices. Join us in shaping the future of sustainable chemistry and engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


