1,7-Dihalogenated BODIPYs: Synthesis, Structure and Photophysics
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
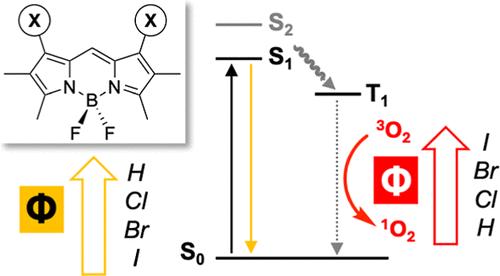
4,4-Difluoro-4-bora-3a,4a-diaza-s-indacene (BODIPY) and its derivatives are highly useful fluorescent dyes employed in myriad applications in chemistry and biology. Here, we revisit a series of dihalogenated (Cl, Br, I) BODIPY derivatives with rare 1,7-regiochemistry. In addition to their synthesis and structural characterization, we fill in a missing piece of the current literature by delineating their photophysical behavior, including the light-driven generation of singlet oxygen (1O2) which is mediated with particularly high efficiency by the heavier diiodinated congener.
1,7-二卤化体dipys:合成、结构及光物理
4,4-二氟-4-硼-3a,4 -二氮-s-茚二烯(BODIPY)及其衍生物是一种非常有用的荧光染料,在化学和生物学中有着广泛的应用。在这里,我们回顾了一系列具有罕见的1,7-区域化学的二卤化(Cl, Br, I) BODIPY衍生物。除了它们的合成和结构表征外,我们还通过描述它们的光物理行为来填补当前文献中缺失的一部分,包括由较重的二碘化同系物以特别高的效率介导的单线态氧(1O2)的光驱动生成。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


