The selective autophagic degradation of avian metapneumovirus subgroup C M2-2 protein via SQSTM1 suppresses viral replication.
Autophagy reports
Pub Date : 2024-09-21
eCollection Date: 2024-01-01
DOI:10.1080/27694127.2024.2375936
引用次数: 0
Abstract
Avian metapneumovirus subgroup C (aMPV/C) is an emerging pathogen that causes acute respiratory infection in chickens and turkeys. Sequestosome 1 (SQSTM1), a selective autophagy receptor, regulates cellular activity or viral replication by recognizing ubiquitinated substrates. Here, we found that SQSTM1 expression inhibits aMPV/C replication through selective autophagy. In particular, SQSTM1 interacts with the aMPV/C M2-2 protein via its PB1 domain, and by recognizing a ubiquitinated lysine at position 67 of viral M2-2 protein. This recognition leads to the autophagic degradation of the aMPV/C M2-2 protein, suppressing viral replication.
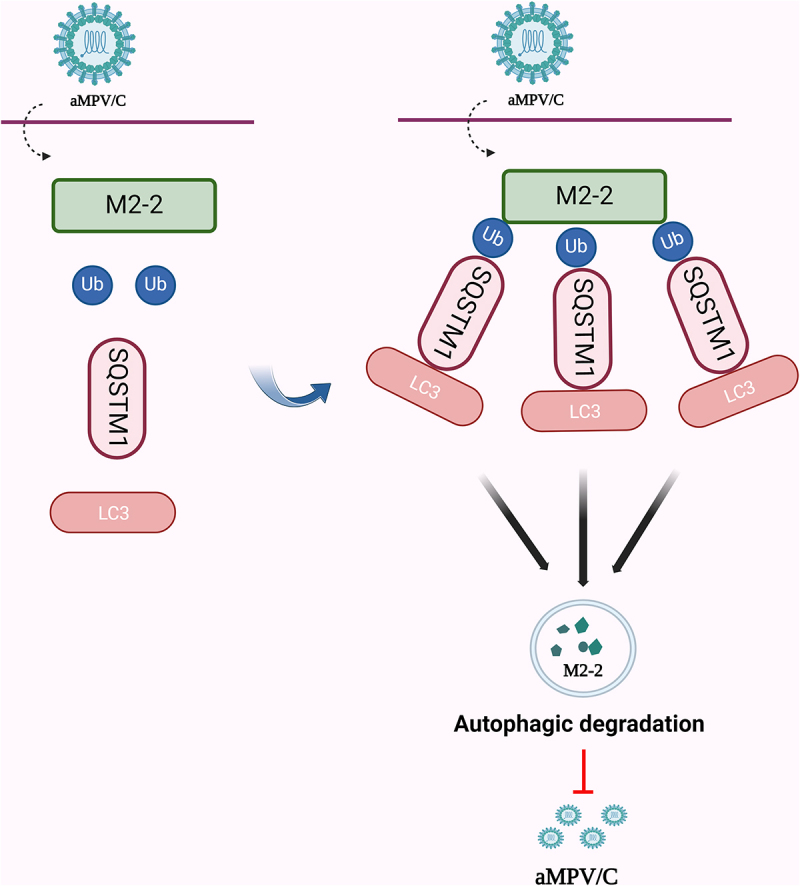
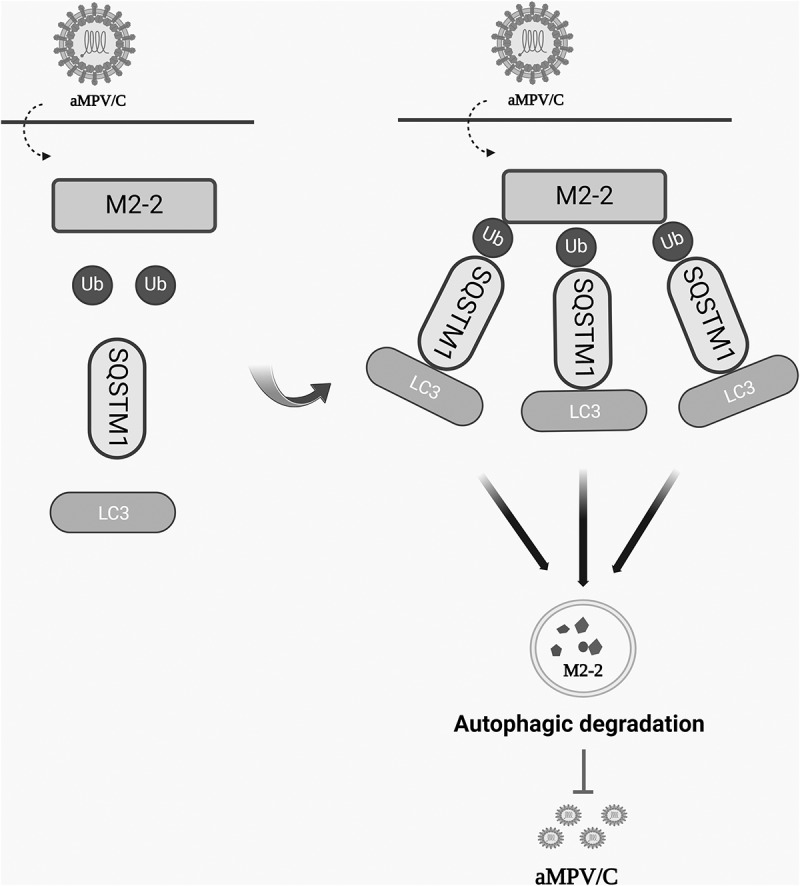
禽偏肺病毒C亚群M2-2蛋白通过SQSTM1选择性自噬降解抑制病毒复制。
禽偏肺病毒亚群C (aMPV/C)是一种引起鸡和火鸡急性呼吸道感染的新兴病原体。Sequestosome 1 (SQSTM1)是一种选择性自噬受体,通过识别泛素化底物来调节细胞活性或病毒复制。在这里,我们发现SQSTM1表达通过选择性自噬抑制aMPV/C复制。特别是,SQSTM1通过其PB1结构域与aMPV/C M2-2蛋白相互作用,并通过识别病毒M2-2蛋白67位的泛素化赖氨酸。这种识别导致aMPV/C M2-2蛋白的自噬降解,抑制病毒复制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


