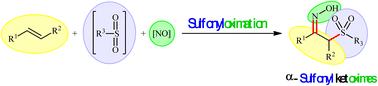
Direct vicinal sulfonyloximation of alkenes: an efficient and straightforward approach towards the synthesis of α-sulfonyl ketoximes
IF 4.6
3区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Direct vicinal sulfonylative difunctionalization of simple alkenes represents a powerful strategy for the rapid assembly of β-functionalized sulfones from simple starting materials. In this context, the direct sulfonyloximation of alkene substrates has recently received much attention from the chemical community owing to important applications of α-sulfonyl ketoxime products in organic synthesis. This review provides an overview of recent research on the titled reactions, with an emphasis on the reaction patterns and mechanisms. Literature has been surveyed until the end of 2024.
烯烃直接邻磺酰肟化:合成α-磺酰酮肟的一种简单有效的方法
简单烯烃直接邻磺酰化双功能化是一种从简单原料快速组装β功能化砜的有效方法。在此背景下,由于α-磺酰酮肟在有机合成中的重要应用,烯烃底物的直接磺酰肟化近年来受到了化学界的广泛关注。本文综述了近年来这类反应的研究进展,重点介绍了反应模式和机理。文献调查一直持续到2024年底。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

RSC Advances
chemical sciences-
CiteScore
7.50
自引率
2.60%
发文量
3116
审稿时长
1.6 months
期刊介绍:
An international, peer-reviewed journal covering all of the chemical sciences, including multidisciplinary and emerging areas. RSC Advances is a gold open access journal allowing researchers free access to research articles, and offering an affordable open access publishing option for authors around the world.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


