The complex world of eutectic solvents: Guidelines for a correct characterization and use in sample preparation
IF 6.5
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
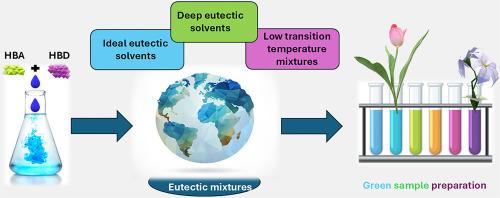
Mixtures with low-temperature phase transitions have been known since the late 19th century, when the term “eutaxia” was first introduced. A major turning point came in 2003, when Abbott and colleagues coined the term “deep eutectic solvent” (DES) to describe mixtures whose melting points are significantly lower than those of their individual components. Since then, these systems have attracted growing interest and have widely been applied across various areas of Chemistry. However, the term DES has often been misused due to the lack of a strict and clear definition for many years. This has led to confusion among DESs (non-ideal systems), eutectic solvents (ESs, thermodynamically ideal systems), and low transition temperature mixtures (LTTMs, metastable systems). This ambiguity is especially pronounced in Analytical Chemistry, where the term DES is frequently used as a catch-all label for not-well characterized mixtures. In many studies, practical applications have been prioritized over a thorough understanding of the physicochemical nature of these solvents. As a result, essential thermodynamic data are often lacking, which hinders the rational design of new systems and the selection of optimal solvents for specific purposes, particularly in extraction processes.
This review aims to clarify the terminology and classification of ESs, DESs, and LTTMs through representative examples and practical applications. It traces the historical development of these unique solvents and highlights their strengths, limitations, and versatility in sample preparation. Special emphasis is placed on the role of water in tuning extraction efficiency and on the use of computational tools to design task-specific DESs. Helpful guidelines are proposed for a proper characterization leading to an precise classification and effective application.
共晶溶剂的复杂世界:在样品制备中正确表征和使用的指南
具有低温相变的混合物早在19世纪末就已为人所知,当时首次引入了“优生态”一词。一个重要的转折点出现在2003年,当时雅培和他的同事们创造了“深共晶溶剂”(DES)这个术语,用来描述熔点明显低于其单个组分的混合物。从那时起,这些系统吸引了越来越多的兴趣,并被广泛应用于化学的各个领域。然而,由于多年来缺乏严格和明确的定义,术语DES经常被误用。这导致了DESs(非理想体系)、共晶溶剂(ESs,热力学理想体系)和低转变温度混合物(LTTMs,亚稳体系)之间的混淆。这种歧义在分析化学中尤其明显,在分析化学中,术语DES经常被用作不完全表征的混合物的包罗万象的标签。在许多研究中,实际应用已经优先于彻底了解这些溶剂的物理化学性质。因此,通常缺乏基本的热力学数据,这妨碍了合理设计新系统和为特定目的选择最佳溶剂,特别是在萃取过程中。本文旨在通过具有代表性的例子和实际应用,阐明ESs、DESs和lttm的术语和分类。它追溯了这些独特溶剂的历史发展,并突出了它们在样品制备中的优势,局限性和多功能性。特别强调了水在调整提取效率方面的作用,以及使用计算工具来设计特定任务的DESs。提出了有用的指导方针,以正确的表征导致精确的分类和有效的应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


