Phase-selective synthesis of 2H-MoTe₂ via alkali-chalcogen exchange
IF 6.2
3区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
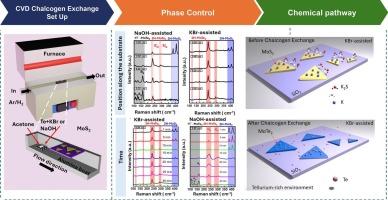
In this study, we explore the phase-selective synthesis of MoTe₂ through alkali-chalcogen exchange in MoS₂ crystals, facilitated by two alkali compounds: NaOH and KBr. The aim is to achieve the structural transformation from 2H-MoS₂ to 2H-MoTe₂ by atomic substitution of sulfur with tellurium. Our results demonstrate that at 500 °C, NaOH induces an uneven formation of both the 1 T’ and 2H phases of MoTe₂, whereas at 750 °C, KBr promotes the exclusive and homogeneous formation of the 2H phase. Raman spectroscopy, X-ray photoelectron spectroscopy, atomic force microscopy and scanning electron microscopy were used to characterize the resulting crystals. The KBr-assisted method results in a more uniform phase transition with higher crystallinity and better stability compared to the NaOH-assisted method, which also induces the formation of oxide and Na![]() O interaction. This study proposes a reaction pathway for KBr assisted process and highlights the advantages of KBr in achieving 2H phase-pure MoTe₂ through density functional theory calculations, which could create new opportunities for research in material engineering and its use in optoelectronic devices.
O interaction. This study proposes a reaction pathway for KBr assisted process and highlights the advantages of KBr in achieving 2H phase-pure MoTe₂ through density functional theory calculations, which could create new opportunities for research in material engineering and its use in optoelectronic devices.
碱-硫交换相选择合成2H-MoTe 2
在这项研究中,我们探索了在NaOH和KBr两种碱化合物的促进下,通过MoS 2晶体中的碱-硫交换来相选择性合成MoTe 2。目的是通过用碲原子取代硫,实现从2H-MoS 2到2H-MoTe 2的结构转变。我们的研究结果表明,在500°C时,NaOH诱导MoTe₂的1t′和2H相的不均匀形成,而在750°C时,KBr促进2H相的均匀形成。利用拉曼光谱、x射线光电子能谱、原子力显微镜和扫描电镜对所得晶体进行了表征。与naoh辅助方法相比,kbr辅助方法的相变更均匀,结晶度更高,稳定性更好,同时也诱导了氧化物的形成和NaO的相互作用。本研究提出了KBr辅助工艺的反应途径,并通过密度泛函理论计算强调了KBr在实现2H相纯MoTe₂方面的优势,为材料工程及其在光电器件中的应用研究创造了新的机会。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

FlatChem
Multiple-
CiteScore
8.40
自引率
6.50%
发文量
104
审稿时长
26 days
期刊介绍:
FlatChem - Chemistry of Flat Materials, a new voice in the community, publishes original and significant, cutting-edge research related to the chemistry of graphene and related 2D & layered materials. The overall aim of the journal is to combine the chemistry and applications of these materials, where the submission of communications, full papers, and concepts should contain chemistry in a materials context, which can be both experimental and/or theoretical. In addition to original research articles, FlatChem also offers reviews, minireviews, highlights and perspectives on the future of this research area with the scientific leaders in fields related to Flat Materials. Topics of interest include, but are not limited to, the following: -Design, synthesis, applications and investigation of graphene, graphene related materials and other 2D & layered materials (for example Silicene, Germanene, Phosphorene, MXenes, Boron nitride, Transition metal dichalcogenides) -Characterization of these materials using all forms of spectroscopy and microscopy techniques -Chemical modification or functionalization and dispersion of these materials, as well as interactions with other materials -Exploring the surface chemistry of these materials for applications in: Sensors or detectors in electrochemical/Lab on a Chip devices, Composite materials, Membranes, Environment technology, Catalysis for energy storage and conversion (for example fuel cells, supercapacitors, batteries, hydrogen storage), Biomedical technology (drug delivery, biosensing, bioimaging)
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


