Dissolved Fe species enable a cooperative solid–molecular mechanism for the oxygen evolution reaction on NiFe-based catalysts
IF 44.6
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
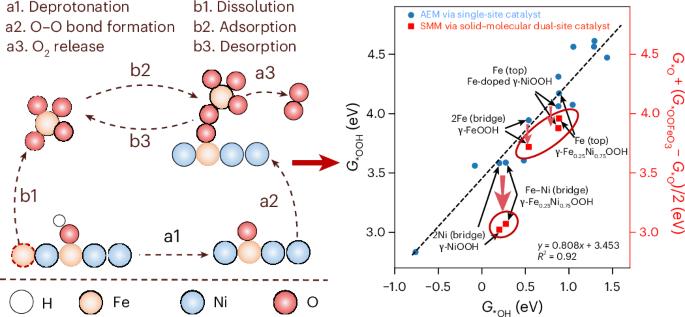
The oxygen evolution reaction is a key process in many energy technologies, but improving its efficiency remains challenging due to the energy scaling relationships that limit the reaction kinetics on conventional single-active-site solid catalysts. Here we report a cooperative solid–molecular mechanism for oxygen evolution on NiFe-based hydroxide electrocatalysts. By identifying the critical interfacial species and understanding their dynamics, we find that molecular FeO42− species, derived from the dissolution of Fe from the solid catalyst, act as molecular co-catalysts that participate in the critical O–O bond-formation step along with solid sites. This synergistic mechanism, involving both solid and molecular active species, circumvents the typical scaling limitations observed for solid catalysts alone. Our findings reveal an unconventional solid–molecular mechanism that governs electrocatalysis at the solid–liquid interface and suggest a strategy for transcending scaling constraints through cooperative multi-site catalysis. NiFe-based catalysts are promising for water oxidation in alkaline electrolytes, but their dynamic structure under operation hinders the establishment of design principles for improved catalytic performance. Now a water oxidation mechanism on mixed NiFe hydroxide catalysts is proposed that involves dissolved FeO42− species acting as co-catalysts.

溶解的铁元素使镍铁基催化剂上的析氧反应具有协同的固-分子机制
析氧反应是许多能源技术中的关键过程,但由于能量缩放关系限制了传统单活性位点固体催化剂的反应动力学,因此提高析氧反应的效率仍然具有挑战性。本文报道了一种基于nife基氢氧化物电催化剂的固-分子协同析氧机制。通过识别关键界面物种并了解其动力学,我们发现FeO42−分子物种,来源于Fe从固体催化剂中溶解,作为分子助催化剂,与固体位点一起参与关键的O-O键形成步骤。这种涉及固体和分子活性物质的协同机制,绕过了仅观察到的固体催化剂的典型结垢限制。我们的发现揭示了一种控制固液界面电催化的非常规固体-分子机制,并提出了一种通过合作多位点催化超越结垢限制的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


