Exercise-induced dysregulation of the adrenergic response in a mouse model of PKP2-arrhythmogenic cardiomyopathy
IF 5.7
2区 医学
Q1 CARDIAC & CARDIOVASCULAR SYSTEMS
引用次数: 0
Abstract
Background
Plakophilin-2 (PKP2) is a component of the desmosome. Pathogenic variants can lead to arrhythmogenic cardiomyopathy (PKP2-ACM). In patients with PKP2-ACM, exercise and catecholamine surges negatively impact arrhythmia incidence and severity.
Objective
The study aimed to characterize remodeling of the sympathetic input and adrenergic response in hearts of PKP2-deficient mice (PKP2cKO) subjected to endurance exercise.
Methods
We used expansion microscopy and structured illumination to characterize the abundance of beta 1-adrenergic receptors (β1-ARs) in the sarcolemma in PKP2cKO-myocytes, sedentary or trained, and Cre-negative controls. We prevented endogenous catecholamine import by short hairpin RNA (shRNA)-mediated knockdown of its transporter [organic cation transporter 3 (OCT3)] and characterized differential effects of isoproterenol (membrane permeable) vs norepinephrine (non-membrane permeable) on Ca2+ transient dynamics. Separately, we evaluated the distribution of sympathetic terminals in PKP2cKO-trained hearts vs controls.
Results
Exercise led to “increased” abundance of sarcolemma β1-ARs in control, and “decreased” abundance in PKP2cKO-myocytes. OCT3 knockdown drastically reduced the response of trained PKP2cKO-myocytes to norepinephrine but not isoproterenol, indicating preserved response to native catecholamines by intracellular (dyad-associated) receptors in the setting of a reduced sarcolemma pool. In tissue, we observed reduced abundance of sympathetic terminals and heterogeneous distribution across the myocardium.
Conclusion
Endurance exercise in PKP2-deficient myocytes leads to a reduced pool of functional β1-ARs in the sarcolemma and yet availability of intracellular receptors, which can activate selected (and heterogeneous) routes of intracellular signaling cascades. We speculate that remodeling of nerve terminals affects sympathetic input distribution and hence, regional modulation of excitability and conduction. These changes can facilitate cell-generated triggered activity and heterogeneity of the underlying substrate, setting the stage for life-threatening arrhythmias.
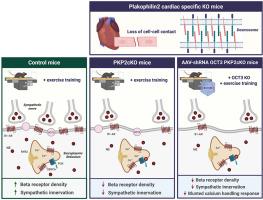
pkp2致心律失常性心肌病小鼠模型运动诱导的肾上腺素能反应失调。
背景:Plakophilin-2 (PKP2)是桥粒的一个组成部分。致病变异可导致心律失常性心肌病(PKP2-ACM)。在PKP2-ACM患者中,运动和儿茶酚胺激增对心律失常的发生率和严重程度有负面影响。目的:探讨耐力运动对pkp2缺陷小鼠(PKP2cKO)心脏交感神经输入和肾上腺素能反应的影响。方法:我们使用扩增显微镜和结构照明来表征pkp2cko肌细胞、久坐或训练以及cre阴性对照中肌膜中β1- ar的丰度。我们通过shrna介导的转运蛋白(OCT3)的敲低来阻止内源性儿茶酚胺的进口,并表征了异丙肾上腺素(膜渗透性)和去甲肾上腺素(非膜渗透性)对Ca2+瞬态动力学的不同影响。另外,我们评估了PKP2cKO训练心脏与对照组交感神经末梢的分布。结果:运动导致对照组肌膜β1- ar丰度升高,而pkp2cko肌细胞中β1- ar丰度降低。OCT3敲除显著降低了训练后的pkp2cko肌细胞对去甲肾上腺素的反应,而不是异丙肾上腺素,这表明在肌膜池减少的情况下,细胞内(双偶体相关)受体对天然儿茶酚胺的反应保持不变。在组织中,我们观察到交感神经末梢的丰度降低,并且在心肌中分布不均。结论:耐力运动导致pkp2缺陷肌细胞中功能性β1- ar库减少,细胞内受体可用性降低,这可以激活细胞内信号级联的选择(和异质)途径。我们推测神经末梢的重塑影响交感神经输入分布,从而影响兴奋性和传导的区域调节。这些变化可以促进细胞产生的触发活性和潜在底物的异质性,为危及生命的心律失常奠定基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Heart rhythm
医学-心血管系统
CiteScore
10.50
自引率
5.50%
发文量
1465
审稿时长
24 days
期刊介绍:
HeartRhythm, the official Journal of the Heart Rhythm Society and the Cardiac Electrophysiology Society, is a unique journal for fundamental discovery and clinical applicability.
HeartRhythm integrates the entire cardiac electrophysiology (EP) community from basic and clinical academic researchers, private practitioners, engineers, allied professionals, industry, and trainees, all of whom are vital and interdependent members of our EP community.
The Heart Rhythm Society is the international leader in science, education, and advocacy for cardiac arrhythmia professionals and patients, and the primary information resource on heart rhythm disorders. Its mission is to improve the care of patients by promoting research, education, and optimal health care policies and standards.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


