A multifunctional endothelial-mimetic surface: Synergistically combating thrombus formation by releasing nitric oxide, promoting fibrinolysis, and enhancing endothelialization
IF 4.7
3区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Thrombus formation often leads to the failure of intravascular implants. Natural endothelium provides multifaceted antithrombotic functions through nitric oxide/ prostacyclin secretion to inhibit platelet activation, glycosaminoglycan mediated anticoagulation, and tissue-type plasminogen activator driven fibrinolysis. Therefore, surfaces mimicking these multiple endothelial functions are expected to have enhanced antithrombotic properties. In this study, polyvinyl chloride surface was rendered porous through solvent/nonsolvent-induced phase separation and loaded with a metal-organic framework, CuBTTri to catalyze nitric oxide release from a precursor. Furthermore, using layer-by-layer self-assembly, multiple bilayers of a poly(lysine-co-oligo(ethylene glycol) methyl ether methacrylate) copolymer (fibrinolysis-promoting), and sodium heparin (endothelial cell growth-promoting), were deposited on the un-etched side of the polyvinyl chloride. This modified surface was shown to be capable of releasing nitric oxide, destroying nascent thrombus, inhibiting smooth muscle cell growth, and promoting endothelial cell adhesion. This study represents a novel approach to developing multifunctional blood-contacting surfaces that mimic multiple properties of the endothelium.
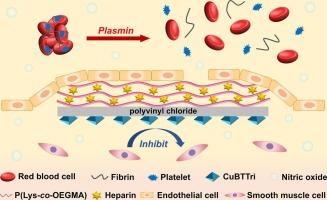
一种多功能的内皮模拟表面:通过释放一氧化氮,促进纤维蛋白溶解和增强内皮化来协同对抗血栓形成
血栓的形成经常导致血管内植入失败。天然内皮通过分泌一氧化氮/前列环素来抑制血小板活化、糖胺聚糖介导的抗凝和组织型纤溶酶原激活剂驱动的纤维蛋白溶解,提供多方面的抗血栓功能。因此,模拟这些多种内皮功能的表面有望具有增强的抗血栓特性。在本研究中,聚氯乙烯表面通过溶剂/非溶剂诱导相分离呈现多孔性,并负载金属-有机骨架cuttri,以催化前驱体释放一氧化氮。此外,通过一层一层的自组装,多层聚赖氨酸-共低聚(乙二醇)甲基醚甲基丙烯酸酯共聚物(促进纤维蛋白溶解)和肝素钠(促进内皮细胞生长)被沉积在聚氯乙烯未蚀刻的一面。这种修饰的表面被证明能够释放一氧化氮,破坏新生血栓,抑制平滑肌细胞生长,促进内皮细胞粘附。这项研究代表了一种开发模拟内皮细胞多种特性的多功能血液接触表面的新方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Colloid and Interface Science Communications
Materials Science-Materials Chemistry
CiteScore
9.40
自引率
6.70%
发文量
125
审稿时长
43 days
期刊介绍:
Colloid and Interface Science Communications provides a forum for the highest visibility and rapid publication of short initial reports on new fundamental concepts, research findings, and topical applications at the forefront of the increasingly interdisciplinary area of colloid and interface science.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


