Boosting energy density of MXenes: Atomically-resolved ion polar rotation within electrolyte-specific-width interlayers
IF 4.2
3区 工程技术
Q2 ELECTROCHEMISTRY
引用次数: 0
Abstract
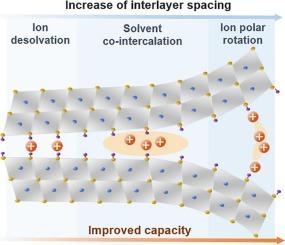
Layer-structured pseudocapacitive electrode materials show excellent potential for overcoming the kinetic constraints of batteries while achieving charge storage capacities greater than those of electrical double-layer capacitors. However, real-world applications are limited by the lack of effective mechanisms to strip electrolyte ions from solvent atoms and control their interactions and charge exchange with the layered electrode materials. Here, we discover the ion polar rotation within sub-nanometer interlayer space of two-dimensional MXene sheets by directly imaging interlayer ionic structure and measuring the local charge transfer. Unlike common ion desolvation, ion polar rotation rearranges solvent molecules and creates more exposure of intercalated ions, eliminating the need for long-range ion diffusion and desolvation. This effect enhances ion-electrode charge transfer at a unique interlayer spacing for each solvent. We demonstrated the efficacy of the polar rotation mechanism by designing a hierarchically structured MXene electrode with the optimal interlayer spacing, which shows Li+ storage capacitance of up to 310 F g−1 (744 F cm−3) in LiTFSI/PC system and good rate performance.
提高MXenes的能量密度:电解质特定宽度夹层内原子分辨离子极性旋转
层结构赝电容电极材料在克服电池动力学约束的同时,实现比电双层电容器更大的电荷存储能力方面显示出优异的潜力。然而,由于缺乏有效的机制来从溶剂原子中剥离电解质离子并控制它们与层状电极材料的相互作用和电荷交换,实际应用受到限制。本文通过直接成像层间离子结构和测量局部电荷转移,发现了二维MXene片亚纳米层间空间内离子的极性旋转。与普通的离子溶解不同,离子极性旋转会重新排列溶剂分子,并产生更多的插入离子,从而消除了远距离离子扩散和溶解的需要。这种效应增强了离子电极在每个溶剂的独特层间距上的电荷转移。我们通过设计具有最佳层间距的分层结构MXene电极,证明了极性旋转机制的有效性,该电极在LiTFSI/PC系统中具有高达310 F g−1 (744 F cm−3)的Li+存储容量和良好的倍率性能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Electrochemistry Communications
工程技术-电化学
CiteScore
8.50
自引率
3.70%
发文量
160
审稿时长
1.2 months
期刊介绍:
Electrochemistry Communications is an open access journal providing fast dissemination of short communications, full communications and mini reviews covering the whole field of electrochemistry which merit urgent publication. Short communications are limited to a maximum of 20,000 characters (including spaces) while full communications and mini reviews are limited to 25,000 characters (including spaces). Supplementary information is permitted for full communications and mini reviews but not for short communications. We aim to be the fastest journal in electrochemistry for these types of papers.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


