Revisiting the TGFβ paradox: insights from HPV-driven cancer and the DNA damage response
IF 66.8
1区 医学
Q1 ONCOLOGY
引用次数: 0
Abstract
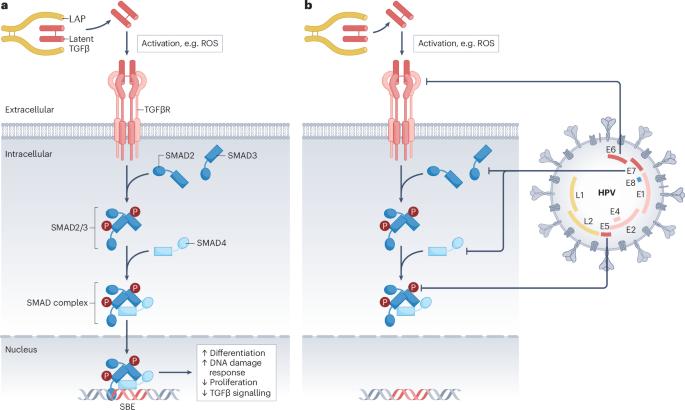
The transforming growth factor-β (TGFβ) paradox refers to the well-established role of TGFβ in suppressing cancer in healthy tissues yet promoting malignancy in established cancers. Although this positioned TGFβ inhibitors as a potential therapeutic strategy for malignancy, therapuetic blockade has failed in multiple clinical trials. The general lack of selection principles for defining which patients would most benefit from the addition of a TGFβ inhibitor has probably hindered its deployment. Here, we highlight the therapeutic potential in TGFβ regulation of DNA repair using human papillomavirus (HPV)-driven head and neck squamous cell carcinoma (HNSCC) as an illustrative example. HPV inhibits TGFβ signalling, which in turn reduces DNA damage repair, ultimately conferring sensitivity to cancer treatments and thus contributing to the favourable prognosis of HPV-positive HNSCC. Here, we review the DNA repair deficit caused by a loss of TGFβ signalling and how this could be targeted to induce synthetic lethality. Moreover, we explore its role in predicting response to immune checkpoint inhibitors and the potential of biomarkers to select which patients with cancer could ultimately benefit from TGFβ inhibition. Transforming growth factor-β (TGFβ) has a well-established role in malignancy and its inhibition has demonstrated strong preclinical activity. However, TGFβ blockade has repeatedly failed in clinical trials. Here, Barcellos-Hoff and Yom utilize human papillomavirus (HPV)-driven cancer as a model to explain these discrepancies and emphasize the need for refined strategies and understanding of TGFβ signalling to enhance therapeutic efficacy.

重新审视TGFβ悖论:来自hpv驱动的癌症和DNA损伤反应的见解
转化生长因子-β (TGFβ)悖论是指TGFβ在健康组织中抑制癌症,但在已建立的癌症中促进恶性肿瘤的既定作用。尽管这将TGFβ抑制剂定位为恶性肿瘤的潜在治疗策略,但治疗阻断在多个临床试验中失败。普遍缺乏选择原则来确定哪些患者将从添加TGFβ抑制剂中获益最多,这可能阻碍了其部署。在这里,我们以人乳头瘤病毒(HPV)驱动的头颈部鳞状细胞癌(HNSCC)为例,强调TGFβ调控DNA修复的治疗潜力。HPV抑制TGFβ信号传导,从而减少DNA损伤修复,最终赋予癌症治疗敏感性,从而有助于HPV阳性HNSCC的良好预后。在这里,我们回顾了由TGFβ信号缺失引起的DNA修复缺陷,以及如何靶向诱导合成致死性。此外,我们探索了它在预测免疫检查点抑制剂反应中的作用,以及生物标志物的潜力,以选择哪些癌症患者最终可以从TGFβ抑制中受益。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Reviews Cancer
医学-肿瘤学
CiteScore
111.90
自引率
0.40%
发文量
97
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Cancer, a part of the Nature Reviews portfolio of journals, aims to be the premier source of reviews and commentaries for the scientific communities it serves. The correct abbreviation for abstracting and indexing purposes is Nat. Rev. Cancer. The international standard serial numbers (ISSN) for Nature Reviews Cancer are 1474-175X (print) and 1474-1768 (online). Unlike other journals, Nature Reviews Cancer does not have an external editorial board. Instead, all editorial decisions are made by a team of full-time professional editors who are PhD-level scientists. The journal publishes Research Highlights, Comments, Reviews, and Perspectives relevant to cancer researchers, ensuring that the articles reach the widest possible audience due to their broad scope.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


