Sensitizing solid tumors to CAR-mediated cytotoxicity by lipid nanoparticle delivery of synthetic antigens
IF 28.5
1区 医学
Q1 ONCOLOGY
引用次数: 0
Abstract
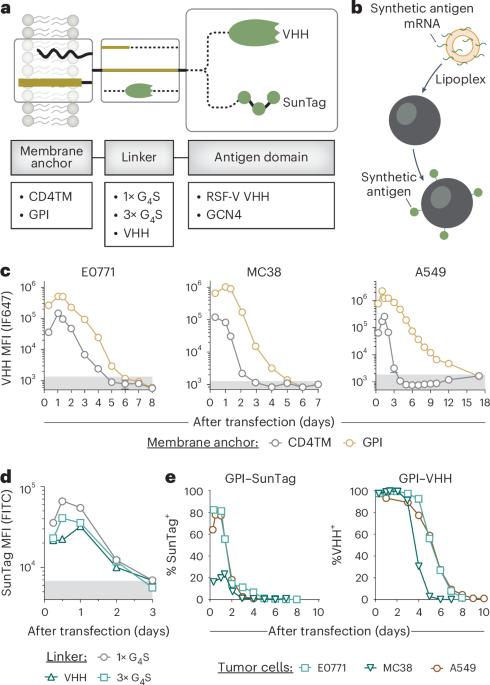
Chimeric antigen receptor (CAR) T cell immunotherapy relies on CAR targeting of tumor-associated antigens; however, heterogenous antigen expression, interpatient variation and off-tumor expression by healthy cells remain barriers. Here we develop synthetic antigens to sensitize solid tumors for recognition and elimination by CAR T cells. Unlike tumor-associated antigens, we design synthetic antigens that are orthogonal to endogenous proteins to eliminate off-tumor targeting and that have a small genetic footprint to facilitate efficient tumor delivery to tumors by lipid nanoparticles. Using a camelid single-domain antibody (VHH) as a synthetic antigen, we show that adoptive transfer of anti-VHH CAR T cells to female mice bearing VHH-expressing tumors reduced tumor burden in multiple syngeneic and xenograft models of cancer, improved survival, induced epitope spread, protected against tumor rechallenge and mitigated antigen escape in heterogenous tumors. Our work supports the in situ delivery of synthetic antigens to treat antigen-low or antigen-negative tumors with CAR T cells. Gamboa et al. develop a chimeric antigen receptor (CAR) T cell therapeutic strategy based on synthetic antigens that are delivered to the tumor by lipid nanoparticles and demonstrate the therapeutic efficacy of their approach using CAR T cells directed against the synthetic antigen.
脂质纳米颗粒递送合成抗原使实体瘤对car介导的细胞毒性敏感。
嵌合抗原受体(CAR) T细胞免疫治疗依赖于CAR靶向肿瘤相关抗原;然而,异质抗原表达、患者间变异和健康细胞的肿瘤外表达仍然是障碍。在这里,我们开发了合成抗原,使实体瘤敏感,以供CAR - T细胞识别和消除。与肿瘤相关抗原不同,我们设计了与内源性蛋白正交的合成抗原,以消除肿瘤外靶向,并且具有小的遗传足迹,以促进脂质纳米颗粒对肿瘤的有效递送。利用骆驼单域抗体(VHH)作为合成抗原,研究人员发现,将抗VHH CAR - T细胞过继转移到携带表达VHH肿瘤的雌性小鼠体内,可以减轻肿瘤的负担,提高肿瘤的生存率,诱导表位扩散,防止肿瘤的再攻击,减轻异质肿瘤中的抗原逃逸。我们的工作支持CAR - T细胞原位递送合成抗原来治疗抗原低或抗原阴性肿瘤。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature cancer
Medicine-Oncology
CiteScore
31.10
自引率
1.80%
发文量
129
期刊介绍:
Cancer is a devastating disease responsible for millions of deaths worldwide. However, many of these deaths could be prevented with improved prevention and treatment strategies. To achieve this, it is crucial to focus on accurate diagnosis, effective treatment methods, and understanding the socioeconomic factors that influence cancer rates.
Nature Cancer aims to serve as a unique platform for sharing the latest advancements in cancer research across various scientific fields, encompassing life sciences, physical sciences, applied sciences, and social sciences. The journal is particularly interested in fundamental research that enhances our understanding of tumor development and progression, as well as research that translates this knowledge into clinical applications through innovative diagnostic and therapeutic approaches. Additionally, Nature Cancer welcomes clinical studies that inform cancer diagnosis, treatment, and prevention, along with contributions exploring the societal impact of cancer on a global scale.
In addition to publishing original research, Nature Cancer will feature Comments, Reviews, News & Views, Features, and Correspondence that hold significant value for the diverse field of cancer research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


