Nb2C MXene functionalized janus amphiphilic membrane manipulates antioxidant, antibacterial and proangiogenic properties to accelerate diabetic wound healing
IF 13.2
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
Diabetic wound treatment remains a worldwide clinical challenge due to key issues, including persistent oxidative stress, bacterial infections and impaired angiogenesis, which impede diabetic wound healing. In this study, a multifunctional Janus amphiphilic membrane (Nb@CCJM) was successfully prepared through a double vitrification process for diabetic wound healing. The layers of the Janus membrane were tightly connected due to the nano-interlocking interface formed between the inner Nb2C MXene-functionalized collagen vitrified membrane and the outer CMC vitrified membrane. Nb@CCJM exhibited excellent biocompatibility, outstanding mechanical properties, and appropriate degradation behavior, effectively scavenging significant amounts of ROS both in vivo and in vitro, thereby reducing oxidative stress in cells and tissues. Additionally, Nb@CCJM exhibited remarkable bactericidal activity to prevent wound infections. Owing to the hydrophilicity of the CMC layer, Nb@CCJM displays asymmetric adhesion; its outer layer effectively prevents tissue adhesion while simultaneously resisting bacterial adherence and avoiding infection. Importantly, Nb@CCJM significantly enhanced the migration and tube formation of vascular endothelial cells in vitro and efficiently promoted angiogenesis and wound healing in diabetic mouse models. Furthermore, mechanistic studies indicate that one of the mechanisms through which Nb@CCJM promotes angiogenesis involves the upregulation of SDF-1α and CXCR4 gene expression, thereby activating the SDF-1α/CXCR4 signaling axis along with its downstream MAPK and PI3K/AKT signaling pathways to facilitate vascular endothelial cell migration and accelerate angiogenesis. In summary, Nb@CCJM was prepared using a straightforward and safe method, effectively scavenged ROS, eliminated bacteria, resisted cell adhesion, and promoted angiogenesis, providing a cost-effective solution for accelerating diabetic wound healing.
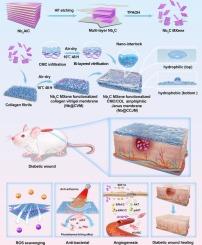
Nb2C MXene功能化的janus两亲膜操纵抗氧化、抗菌和促血管生成特性,加速糖尿病伤口愈合
由于持续氧化应激、细菌感染和血管生成受损等关键问题阻碍了糖尿病伤口愈合,糖尿病伤口治疗仍然是一个全球性的临床挑战。在这项研究中,通过双玻璃化工艺成功制备了一种用于糖尿病伤口愈合的多功能Janus两亲膜(Nb@CCJM)。由于内部Nb2C mxene功能化胶原玻璃化膜与外部CMC玻璃化膜之间形成纳米互锁界面,使Janus膜的各层紧密相连。Nb@CCJM具有优异的生物相容性、优异的机械性能和适当的降解行为,在体内和体外均能有效清除大量ROS,从而减少细胞和组织中的氧化应激。此外,Nb@CCJM显示出显著的杀菌活性,以防止伤口感染。由于CMC层的亲水性,Nb@CCJM呈现不对称粘附;其外层有效防止组织粘连,同时抵抗细菌粘附,避免感染。重要的是,Nb@CCJM显著增强了体外血管内皮细胞的迁移和成管,有效促进了糖尿病小鼠模型的血管生成和伤口愈合。此外,机制研究表明Nb@CCJM促进血管生成的机制之一涉及上调SDF-1α和CXCR4基因表达,从而激活SDF-1α/CXCR4信号轴及其下游的MAPK和PI3K/AKT信号通路,促进血管内皮细胞迁移,加速血管生成。综上所述,Nb@CCJM制备方法简单安全,能有效清除活性氧,消除细菌,抵抗细胞粘连,促进血管生成,为加速糖尿病创面愈合提供了一种高性价比的解决方案。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


