Advances in the mechanisms of HIF-1α-enhanced tumor glycolysis and its relation to dedifferentiation
IF 4.5
3区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
Progress in Biophysics & Molecular Biology
Pub Date : 2025-05-13
DOI:10.1016/j.pbiomolbio.2025.05.003
引用次数: 0
Abstract
Metabolic reprogramming, a hallmark of malignancy, enables tumor cells to adapt to the harsh and dynamic tumor microenvironment (TME) by altering metabolic pathways. Hypoxia, prevalent in solid tumors, activates hypoxia inducible factor 1α (HIF-1α). HIF-1α drives metabolic reprogramming, enhancing glycolysis primarily through the Warburg effect to reduce oxygen dependence and facilitate tumor cell growth/proliferation. The above process is associated with accelerated tumor cell dedifferentiation and enhanced stemness, generating cancer stem cells (CSCs) which possesses the potential for self-renewal and differentiation that can differentiate into a wide range of subtypes of tumor cells and fuel tumor heterogeneity, metastasis, and recurrence, complicating therapy. This review examines the HIF-1α-glycolysis-dedifferentiation crosstalk mechanisms, expecting that indirect inhibition of HIF-1α by targeting metabolic enzymes, metabolites, or their signaling pathways will offer an effective therapeutic strategy to improve the cancer treatment outcomes.
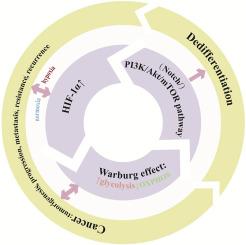
hif -1α-增强肿瘤糖酵解机制及其与去分化关系的研究进展。
代谢重编程是恶性肿瘤的一个标志,它通过改变代谢途径使肿瘤细胞适应恶劣和动态的肿瘤微环境(TME)。缺氧在实体肿瘤中普遍存在,可激活缺氧诱导因子1α (HIF-1α)。HIF-1α驱动代谢重编程,主要通过Warburg效应增强糖酵解,从而降低氧依赖性,促进肿瘤细胞生长/增殖。上述过程与肿瘤细胞去分化加速和干性增强有关,产生具有自我更新和分化潜力的癌症干细胞(cancer stem cells, CSCs),可以分化成广泛的肿瘤细胞亚型,并促进肿瘤异质性、转移和复发,使治疗复杂化。本文综述了HIF-1α-糖酵解-去分化串串机制,期望通过靶向代谢酶、代谢物或其信号通路间接抑制HIF-1α将为改善癌症治疗效果提供有效的治疗策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Progress in Biophysics & Molecular Biology
生物-生化与分子生物学
CiteScore
8.60
自引率
7.90%
发文量
85
审稿时长
85 days
期刊介绍:
Progress in Biophysics & Molecular Biology is an international review journal and covers the ground between the physical and biological sciences since its launch in 1950. It indicates to the physicist the great variety of unsolved problems awaiting attention in biology and medicine. The biologist and biochemist will find that this journal presents new and stimulating ideas and novel approaches to studying and influencing structural and functional properties of the living organism. This journal will be of particular interest to biophysicists, biologists, biochemists, cell physiologists, systems biologists, and molecular biologists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


