Complementary Analysis of Local and Systemic Effects of Dupilumab in Paediatric AD Using Tape Strips and Serum
Abstract
Objective
This study investigates local and systemic immune-related proteins in tape strips and serum of paediatric atopic dermatitis (AD) patients treated with dupilumab, and explores their correlation with clinical severity.
Methods
Twenty paediatric AD patients (< 18 years) starting dupilumab treatment were included. Serum samples and tape strips from lesional and non-lesional skin were collected at baseline, 4 and 16 weeks of treatment. Fifteen pre-specified proteins were measured at each visit by Luminex multiplex immunoassay. Clinical severity outcome measures included the Eczema Area and Severity Index (EASI) and Numeric Rating Scale (NRS) itch. Statistical analyses included Wilcoxon signed-rank tests and Spearman correlations.
Results
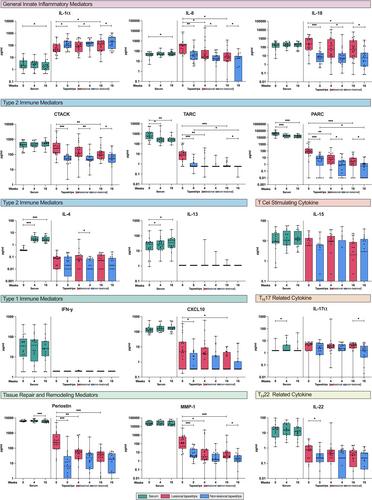
Along with clinical improvement, 16 weeks of dupilumab treatment resulted in a rapid and significant reduction in the disease-associated mediators PARC/CCL18 and TARC/CCL17 in both tape-stripped skin and serum. While the cytokine and chemokine profiles differed between the sampling methods, both effectively captured immunological changes associated with dupilumab treatment. Tape strips demonstrated significant reductions in innate pro-inflammatory cytokines (IL-8/CXCL8, IL-18), the T cell-recruiting chemokine CTACK/CCL27, the Type 1 immune mediator CXCL10, and tissue repair and remodelling proteins (periostin, MMP-1) in response to treatment, but were less sensitive in detecting T cell-derived cytokines (IL-4, IL-13). In both skin and serum, several proteins were significantly correlated with AD severity, as measured by EASI and NRS itch, with PARC/CCL18 emerging as the strongest correlated protein.
Conclusion
Our findings provide insight into the distinct local and systemic proteomic changes in response to dupilumab treatment in paediatric AD patients. These findings underscore the complementary roles of tape strips and serum in profiling immune and epidermal barrier proteins, highlighting the utility of minimally invasive tape stripping for monitoring proteomic responses to targeted therapies in paediatric AD.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


