Edge-N-doped porous graphitic carbon as platinum-based catalyst support for alkaline hydrogen evolution reaction
IF 4.1
3区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
Graphitic carbons are commonly used as supports for electrocatalysts. However, due to their inherent lack of active sites and surface functional groups, graphitic carbon often causes significant aggregation and migration of Pt particles when used as a support for Pt/C catalysts, resulting in decreased activity and stability during the alkaline hydrogen evolution reaction (HER) process. In this study, graphite was first etched with NaOH, followed by reaction with melamine to synthesize edge-N-doped porous graphitic carbon (NPGC) as a support for Pt-based catalysts. The porous structure and edge-N doping promote the dispersion and anchoring of Pt species, while also contributing to the reduction in Pt nanoparticle size. Consequently, the Pt/NPGC catalyst demonstrated a low overpotential of 43 mV at 10 mA·cm−2, outperforming the unmodified Pt/GC (102 mV) and NaOH-etched Pt/PGC (49.7 mV), thus demonstrating superior HER activity. Additionally, the stability test results confirmed the exceptional stability of the Pt/NPGC catalyst. This study provides valuable insights into the design and fabrication of efficient graphitic carbon-supported Pt-based catalysts for potential applications in future hydrogen energy technologies.
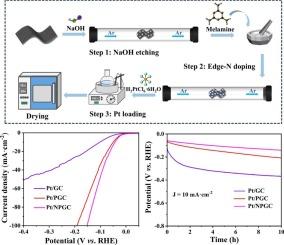
边氮掺杂多孔石墨碳作为碱性析氢反应铂基催化剂载体
石墨碳通常用作电催化剂的载体。然而,由于石墨碳本身缺乏活性位点和表面官能团,在作为Pt/C催化剂的载体时,往往会引起Pt颗粒的明显聚集和迁移,导致碱性析氢反应(HER)过程中的活性和稳定性下降。在这项研究中,石墨首先用NaOH蚀刻,然后与三聚氰胺反应,合成边缘掺杂n的多孔石墨碳(NPGC)作为pt基催化剂的载体。多孔结构和边缘n掺杂促进了Pt的分散和锚定,同时也有助于Pt纳米颗粒尺寸的减小。结果表明,Pt/NPGC催化剂在10 mA·cm−2下的过电位为43 mV,优于未修饰Pt/GC (102 mV)和naoh蚀刻Pt/PGC (49.7 mV),因此具有更好的HER活性。此外,稳定性测试结果证实了Pt/NPGC催化剂的优异稳定性。这项研究为设计和制造高效石墨碳支撑的pt基催化剂提供了有价值的见解,这些催化剂在未来氢能源技术中的潜在应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.80
自引率
6.70%
发文量
912
审稿时长
2.4 months
期刊介绍:
The Journal of Electroanalytical Chemistry is the foremost international journal devoted to the interdisciplinary subject of electrochemistry in all its aspects, theoretical as well as applied.
Electrochemistry is a wide ranging area that is in a state of continuous evolution. Rather than compiling a long list of topics covered by the Journal, the editors would like to draw particular attention to the key issues of novelty, topicality and quality. Papers should present new and interesting electrochemical science in a way that is accessible to the reader. The presentation and discussion should be at a level that is consistent with the international status of the Journal. Reports describing the application of well-established techniques to problems that are essentially technical will not be accepted. Similarly, papers that report observations but fail to provide adequate interpretation will be rejected by the Editors. Papers dealing with technical electrochemistry should be submitted to other specialist journals unless the authors can show that their work provides substantially new insights into electrochemical processes.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


