Biocontrol potential of Vibrio maritimus chitinase: Heterologous expression and insecticidal activity against Acanthoscelides obtectus
IF 8.5
1区 化学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
International Journal of Biological Macromolecules
Pub Date : 2025-05-16
DOI:10.1016/j.ijbiomac.2025.144285
引用次数: 0
Abstract
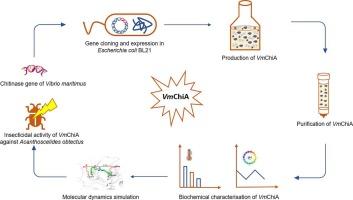
In this study, the chitinase gene from the marine bacterium Vibrio maritimus was heterologously expressed in Escherichia coli, purified via affinity chromatography and tested for its insecticidal activity against the storage pest Acanthoscelides obtectus. The recombinant VmChiA protein exhibited a molecular mass of ∼60 kDa, with optimum activity observed at pH 6.0 and 40 °C. Enzyme kinetic analysis revealed a Km value of 0.042 mM, Vmax of 17.48 μmol min−1, kcat of 1.75 min−1 and catalytic efficiency of 41.61 mM−1 min−1, respectively. Furthermore, a dose of 40 U mL−1 of recombinant VmChiA showed similar efficacy to malathion insecticide against A. obtectus, with 100 % mortality in both treatments. LC50 and LC90 values of VmChiA were 13.95 U mL−1 and 27.66 U mL−1, respectively. Furthermore, the three-dimensional structure of the catalytic site of VmChiA was modeled. Molecular dynamics simulation technique was used to explore and analyze the dynamics and interactions. A salt bridge (GLU274-ARG296) in the α + β domain was observed as a critical feature facilitating substrate (GlcNAc)2 binding and enzymatic activity. These findings demonstrate that recombinant VmChiA possesses potent insecticidal properties, highlighting its potential as a bio-based, eco-friendly alternative for managing significant agricultural pests.
海洋弧菌几丁质酶的生物防治潜力:异源表达及对棘皮虫的杀虫活性
本研究将海洋弧菌的几丁质酶基因在大肠杆菌中异种表达,通过亲和层析纯化,并检测其对储粮害虫棘皮虫的杀虫活性。重组VmChiA蛋白分子量约为60 kDa,在pH 6.0和40°C条件下具有最佳活性。酶动力学分析表明,Km值为0.042 mM, Vmax值为17.48 μmol min−1,kcat值为1.75 min−1,催化效率为41.61 mM−1 min−1。此外,40 U mL−1剂量的重组VmChiA与马拉硫磷杀虫剂对伊蚊的杀伤效果相似,两种处理的死亡率均为100%。VmChiA的LC50和LC90值分别为13.95 U mL−1和27.66 U mL−1。此外,还建立了VmChiA催化位点的三维结构模型。利用分子动力学模拟技术对其动力学和相互作用进行了探索和分析。α + β结构域的盐桥(GLU274-ARG296)是促进底物(GlcNAc)2结合和酶活性的关键特征。这些发现表明,重组VmChiA具有强大的杀虫特性,突出了其作为生物基、生态友好的农业害虫管理替代品的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
13.70
自引率
9.80%
发文量
2728
审稿时长
64 days
期刊介绍:
The International Journal of Biological Macromolecules is a well-established international journal dedicated to research on the chemical and biological aspects of natural macromolecules. Focusing on proteins, macromolecular carbohydrates, glycoproteins, proteoglycans, lignins, biological poly-acids, and nucleic acids, the journal presents the latest findings in molecular structure, properties, biological activities, interactions, modifications, and functional properties. Papers must offer new and novel insights, encompassing related model systems, structural conformational studies, theoretical developments, and analytical techniques. Each paper is required to primarily focus on at least one named biological macromolecule, reflected in the title, abstract, and text.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


