Effect of calcination temperature on the performance of K-Co/Al2O3 catalyst for oxidative coupling of methane
IF 7.5
3区 环境科学与生态学
Q2 ENERGY & FUELS
引用次数: 0
Abstract
The oxidative coupling of methane (OCM) involves directly converting methane to C2+ hydrocarbons (such as ethylene and ethane) via a reaction with oxygen. This study elucidated the effect of the calcination temperature on the structure and catalytic performance of potassium-doped-cobalt oxide supported on an alumina (K-Co/Al2O3) catalyst for the OCM reaction. The catalyst was highly active at relatively low reactor temperatures (500–640 °C). Four calcination temperatures (400, 500, 600, and 700 °C) were investigated, with the results showing that the calcination temperature strongly affected catalytic properties, such as the crystalline phases, elemental distribution, physical properties, and catalytic basicity, leading to a wide range in catalytic performances. The catalyst calcined at 400 °C was superior among the catalysts, with 8.3 % C2+ yield, 24.8 % C2+ selectivity, and 33.6 % CH4 conversion at 640 °C. Furthermore, the catalyst was robust over 24 h of testing.
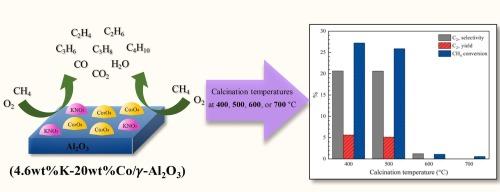
煅烧温度对K-Co/Al2O3甲烷氧化偶联催化剂性能的影响
甲烷的氧化偶联(OCM)涉及通过与氧的反应将甲烷直接转化为C2+碳氢化合物(如乙烯和乙烷)。研究了煅烧温度对负载在氧化铝(K-Co/Al2O3)催化剂上的掺钾氧化钴的结构和催化性能的影响。催化剂在较低的反应温度(500-640℃)下具有较高的活性。研究了400、500、600和700℃四种煅烧温度,结果表明,煅烧温度对催化剂的晶相、元素分布、物理性质和催化碱度等催化性能有较大影响,导致催化剂的催化性能变化较大。400℃煅烧催化剂的C2+产率为8.3%,C2+选择性为24.8%,CH4转化率为33.6%。此外,在24小时的测试中,催化剂具有很强的鲁棒性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Carbon Resources Conversion
Materials Science-Materials Science (miscellaneous)
CiteScore
9.90
自引率
11.70%
发文量
36
审稿时长
10 weeks
期刊介绍:
Carbon Resources Conversion (CRC) publishes fundamental studies and industrial developments regarding relevant technologies aiming for the clean, efficient, value-added, and low-carbon utilization of carbon-containing resources as fuel for energy and as feedstock for materials or chemicals from, for example, fossil fuels, biomass, syngas, CO2, hydrocarbons, and organic wastes via physical, thermal, chemical, biological, and other technical methods. CRC also publishes scientific and engineering studies on resource characterization and pretreatment, carbon material innovation and production, clean technologies related to carbon resource conversion and utilization, and various process-supporting technologies, including on-line or off-line measurement and monitoring, modeling, simulations focused on safe and efficient process operation and control, and process and equipment optimization.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


