Determination of the composition and monosaccharide content of atractylodes polysaccharides using pre-column derivatization and a quantitative analysis of multicomponents by a single marker method
IF 3.2
引用次数: 0
Abstract
Atractylodes chinensis (DC.) Koidz. (ACK), a prominent herb in traditional Chinese medicine, is widely used to treat conditions such as dampness obstruction in the middle Jiao, epigastric distension, diarrhea, and edema. The primary bioactive components of ACK include sesquiterpenes, alkynes, triterpenes, and polysaccharides. In this study, a high-performance liquid chromatography (HPLC) method with pre-column derivatization using 1-phenyl-3-methyl-5-pyrazolone (PMP) was developed. Additionally, a quantitative analysis of multicomponents by a single marker (QAMS) method was established to quantify polysaccharide components in ACK with high precision. Atractylodes chinensis polysaccharides (ACP) underwent acid hydrolysis with hydrochloric acid, yielding stable monosaccharide-PMP derivatives through pre-column derivatization. These derivatives were analyzed by HPLC, with anhydrous glucose as the internal standard. The relative correction factors for glucuronic acid, galactose, and arabinose were calculated using the QAMS method, yielding values of 1.08, 0.97, and 0.75, respectively. The monosaccharide contents determined by QAMS showed no significant difference from those obtained by the external standard method (ESM), with all relative standard deviations (RSD%) <2 %. This method exhibited good linearity—linear ranges: glucuronic acid (0.0250–0.1250 mg/mL, r = 0.9996), anhydrous glucose (0.0094–0.0472 mg/mL, r = 0.9996), galactose (0.0100–0.0500 mg/mL, r = 0.9997), arabinose (0.0250–0.1250 mg/mL, r = 0.9987)—and the RSD% values for the stability of these four components within 48 hours were all <3 %. In terms of reproducibility, multiple injections (n = 6) showed low relative standard deviations (RSD%): glucuronic acid (2.04 %), anhydrous glucose (0.32 %), galactose (0.53 %), arabinose (0.53 %). Regarding stability, within [specific time range], the RSD% values were: glucuronic acid (1.71 %), anhydrous glucose (2.49 %), galactose (2.11 %), arabinose (1.76 %). The accuracy was also satisfactory, with an RSD% of 3.45 %. These findings indicate that the established approach is robust, reliable, and applicable for determining monosaccharide content in hydrolyzed ACP. This methodology provides a promising tool for quality control and further pharmacological studies of ACP.
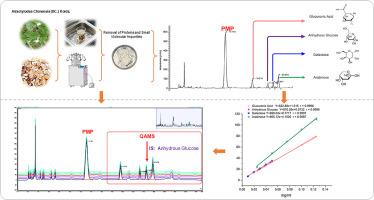
柱前衍生法测定苍术多糖的组成和单糖含量,单标记法测定多组分
白术(苍术)Koidz。(ACK)是一种著名的中药,广泛用于治疗中焦湿阻、胃脘胀、腹泻、水肿等病症。ACK的主要生物活性成分包括倍半萜、炔烃、三萜和多糖。本研究建立了以1-苯基-3-甲基-5-吡唑酮(PMP)为原料柱前衍生的高效液相色谱(HPLC)方法。此外,建立了单标记多组分定量分析(QAMS)方法,对ACK中的多糖成分进行了高精度定量。摘要白术多糖(ACP)经盐酸酸水解,柱前衍生得到稳定的单糖- pmp衍生物。采用高效液相色谱法,以无水葡萄糖为内标进行分析。采用QAMS法计算葡萄糖醛酸、半乳糖和阿拉伯糖的相对校正因子,分别为1.08、0.97和0.75。QAMS法测定的单糖含量与外标法(ESM)测定的单糖含量无显著差异,相对标准偏差(RSD%)均为2%。该方法具有良好的线性范围:葡萄糖醛酸(0.0250 ~ 0.1250 mg/mL, r = 0.9996)、无水葡萄糖(0.0094 ~ 0.0472 mg/mL, r = 0.9996)、半乳糖(0.0100 ~ 0.0500 mg/mL, r = 0.9997)、阿拉伯糖(0.0250 ~ 0.1250 mg/mL, r = 0.9987), 4种成分在48 h内稳定性的RSD%值均为3%。在重复性方面,多次注射(n = 6)具有较低的相对标准偏差(RSD%):葡萄糖醛酸(2.04%)、无水葡萄糖(0.32%)、半乳糖(0.53%)、阿拉伯糖(0.53%)。稳定性方面,在特定时间范围内,RSD%分别为:葡萄糖醛酸(1.71%)、无水葡萄糖(2.49%)、半乳糖(2.11%)、阿拉伯糖(1.76%)。准确度也令人满意,RSD%为3.45%。结果表明,所建立的方法稳健、可靠,可用于测定水解ACP中单糖的含量。该方法为ACP的质量控制和进一步的药理研究提供了一个有前途的工具。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of chromatography open
Analytical Chemistry
CiteScore
2.50
自引率
0.00%
发文量
0
审稿时长
50 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


