In Situ Generation and [3 + 2] Annulation Reactions of Propiolaldehyde─A Metal-Free, Cascade Route to Pyrazole and Bipyrazole Carboxaldehydes in One Pot
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
Journal of Organic Chemistry
Pub Date : 2025-05-07
DOI:10.1021/acs.joc.5c0024610.1021/acs.joc.5c00246
引用次数: 0
Abstract
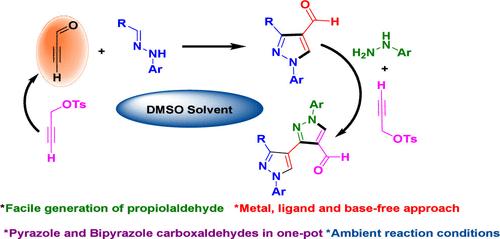
Propiolaldehyde, the most reactive yet less explored electrophilic acetylene, was generated in situ via a base-free Kornblum oxidation from propargyl tosylate and successfully intercepted with hydrazones affording pyrazole-4-carboxaldehyde in one-pot by a [3 + 2] annulation reaction. Further, the pyrazole-4-carboxaldehyde endured a unique cascade reaction with phenylhydrazine and propargyl tosylate, yielding synthetically challenging bipyrazole carboxaldehydes. The method is free of any metal catalyst, base, or additives and offers a convenient protocol to handle the reactive and volatile propiolaldehyde under ambient conditions.
丙醛的原位生成和[3 + 2]环化反应─一种无金属级联制吡唑和联吡唑羧醛的途径
丙醛是一种活性最高但研究较少的亲电性乙炔,它通过无碱的Kornblum氧化从丙炔酸酯中原位生成,并通过[3 + 2]环化反应成功地与产生吡唑-4-甲醛的腙体进行了阻断。此外,吡唑-4-甲醛与苯肼和丙炔甲酯发生了独特的级联反应,生成了具有合成挑战性的联吡唑甲醛。该方法不含任何金属催化剂、碱或添加剂,为在环境条件下处理反应性和挥发性丙醛提供了方便的方案。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


