Electrochemically Induced Synthesis of Substituted 5-Thiotetrazoles
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
Journal of Organic Chemistry
Pub Date : 2025-05-06
DOI:10.1021/acs.joc.4c0282210.1021/acs.joc.4c02822
引用次数: 0
Abstract
A novel electrochemical procedure for the one-pot synthesis of variously substituted 5-thiotetrazole has been established by a three-component reaction (thiol(thiophenol)s, isocyanides, and azidotrimethylsilane). This approach is achieved by the sulfidation of isocyanide and subsequent cycloaddition with azidotrimethylsilane under mild conditions without a metal catalyst or external oxidant. Importantly, the presented methodology not only features a wide substrate scope but also exhibits the practicality showcased by gram-scale synthesis and various conversions.
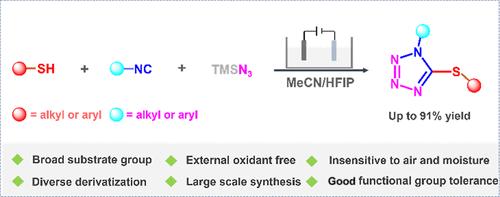
电化学诱导合成取代5-硫代四唑
通过三组分反应(硫醇(噻吩)s、异氰酸酯和叠氮三甲基硅烷),建立了一锅法合成不同取代的5-硫代四唑的电化学新方法。该方法是在温和的条件下,在没有金属催化剂或外部氧化剂的情况下,通过异氰化物的硫化和随后的叠氮三甲基硅烷的环加成来实现的。重要的是,所提出的方法不仅具有广泛的衬底范围,而且具有克尺度合成和各种转换所展示的实用性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


