The synergistic effect between Ru single-atomic sites and nanoclusters during catalytic hydrotreatment of fast pyrolysis liquids from lignocellulosic biomass
IF 4.3
2区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
Catalytic hydrotreatment is a promising technique to upgrade pyrolysis liquids (PLs) with undesired properties into intermediate that can be co-processed with vacuum gas oil in FCC refinery. Highly active catalysts are key in such a process. In this study, a ruthenium-based catalyst possessing both single-atomic and nanocluster sites supported on hierarchically porous nitrogen-doped carbon (Ru1+NPs/HPNC) was prepared by a two-step alcohol reduction method. Catalytic performance was evaluated for both model compound vanillin (VL) and PLs in a batch autoclave. The model compound study showed that Ru1+NPs/HPNC exhibited excellent intrinsic activity in VL hydrogenation, with a TOF of 26.9 s−1, which is approximately 3 times higher than that of Ru1/HPNC (8.4 s−1) and RuNPs/HPNC (8.8 s−1), and 6 times that of Ru/AC (4.2 s−1). Catalytic hydrotreatment of PLs indicated that Ru1+NPs/HPNC possessed the best activity regarding to the highest H/C ratio (mild hydrotreatment: 1.33; deep hydrotreatment: 1.29) and the lowest TG residue (mild hydrotreatment: 14.4 wt%; deep hydrotreatment: 6.5 wt%) of the product oils. To obtain understanding of the synergistic effect between single-atoms and nanoclusters, the adsorption of VL and H2 on the catalyst was examined by ATR-FTIR and DFT calculations. The results revealed that VL is preferentially adsorbed and activated on the single-atomic sites, while the H2 is preferentially dissociated on the nanocluster sites. Based on these findings, a plausible mechanism is proposed. This study offers new ideas for developing better-performing catalysts for catalytic hydrotreatment of PLs.
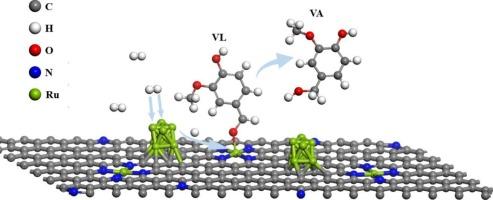
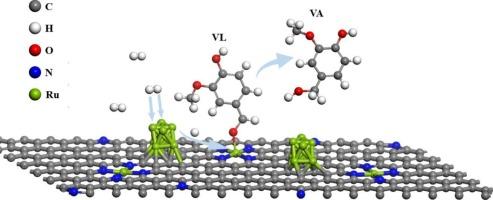
Ru单原子位与纳米团簇在催化加氢处理木质纤维素生物质快热解液中的协同作用
催化加氢处理是一种很有前途的技术,它可以将不需要的热解液转化为可与催化裂化炼油厂的真空瓦斯油协同处理的中间体。高活性催化剂是这一过程的关键。在本研究中,采用两步醇还原法制备了一种钌基催化剂,该催化剂具有单原子和纳米簇位点,负载在分层多孔氮掺杂碳(Ru1+NPs/HPNC)上。在间歇式热压釜中对模型化合物香兰素(VL)和PLs的催化性能进行了评价。模型化合物研究表明,Ru1+NPs/HPNC具有良好的VL加氢活性,TOF为26.9 s−1,约为Ru1/HPNC(8.4 s−1)和RuNPs/HPNC(8.8 s−1)的3倍,是Ru/AC(4.2 s−1)的6倍。PLs的催化加氢处理表明,Ru1+NPs/HPNC具有最佳的活性,H/C比最高(轻度加氢:1.33;深度加氢处理:1.29)和最低TG残留物(轻度加氢处理:14.4 wt%;深度加氢处理:6.5 wt%)的产品油。为了了解单原子和纳米团簇之间的协同作用,通过ATR-FTIR和DFT计算考察了催化剂对VL和H2的吸附。结果表明,VL在单原子位点上优先被吸附和活化,而H2在纳米簇位点上优先被解离。基于这些发现,我们提出了一个合理的机制。本研究为开发性能更好的PLs催化加氢处理催化剂提供了新的思路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Science
工程技术-工程:化工
CiteScore
7.50
自引率
8.50%
发文量
1025
审稿时长
50 days
期刊介绍:
Chemical engineering enables the transformation of natural resources and energy into useful products for society. It draws on and applies natural sciences, mathematics and economics, and has developed fundamental engineering science that underpins the discipline.
Chemical Engineering Science (CES) has been publishing papers on the fundamentals of chemical engineering since 1951. CES is the platform where the most significant advances in the discipline have ever since been published. Chemical Engineering Science has accompanied and sustained chemical engineering through its development into the vibrant and broad scientific discipline it is today.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


