High performance-green extraction of base metals (Zn and Pb) and gold from polymetallic sulfide concentrate using p-toluenesulfonic acid-based deep eutectic solvents
IF 9
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
Sulfide minerals are crucial for metal supply, but their extraction traditionally relies on energy-intensive and environmentally harmful methods. Deep Eutectic Solvents (DESs) offer a promising alternative for sustainable metal extraction. This study investigates the feasibility of using DESs to leach lead, zinc, and gold from a polymetallic sulfide concentrate. The sample is primarily composed of sphalerite, galena, chalcopyrite, and pyrite. Five DESs were synthesized and evaluated for their leaching efficiency. The results showed that choline chloride (ChCl) as a hydrogen bond acceptor and p-toluenesulfonic acid (PTSA) as a hydrogen bond donor play significant roles in dissolving metals. Further experiments with the most effective deep eutectic solvents (DESs), namely D1, D2, and D3, were conducted to evaluate the influence of temperature on leaching efficiency. The DES composed of 1PEG-400:1PTSA (D1) demonstrated a high capacity for selectively solubilizing lead (Pb), achieving 100 % recovery, gold (Au) recovery reached 55 % and zinc recovery only 6 % under optimal conditions. The DES composed of 2ChCl:1PTSA (D2) exhibited strong ability in dissolving both Pb and Zn, achieving nearly 100 % recovery for each, along with 73 % recovery for Au. Lastly, the DES consisting of 1ChCl:2Acetic acid (D3) also performed well in dissolving Pb, with a recovery rate exceeding 86 % but zinc recovery reached 4 %. The dissolution of lead is predominantly diffusion-controlled, with activation energies ranging from 24 to 34 kJ/mol. For zinc, lower activation energies 22–32 kJ/mol suggest a transitional regime between diffusion and chemical control. Neither lead nor zinc dissolution exceed the 40 kJ/mol threshold for chemical control.
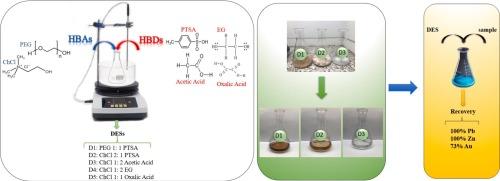
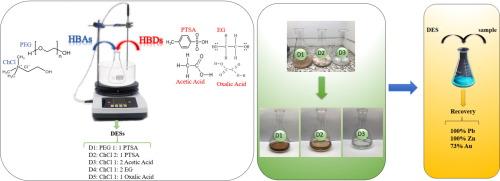
采用对甲苯磺酸基深共晶溶剂萃取多金属硫化物精矿中的贱金属(锌、铅)和金
硫化物矿物对金属供应至关重要,但它们的提取传统上依赖于能源密集型和对环境有害的方法。深共晶溶剂(DESs)是一种很有前途的可持续金属萃取方法。本研究探讨了用DESs浸出某多金属硫化精矿中铅、锌、金的可行性。样品主要由闪锌矿、方铅矿、黄铜矿和黄铁矿组成。合成了5种DESs,并对其浸出效率进行了评价。结果表明,氯化胆碱(ChCl)作为氢键受体,对甲苯磺酸(PTSA)作为氢键给体对金属的溶解起重要作用。采用最有效的深度共晶溶剂(DESs) D1、D2和D3进行进一步实验,以评估温度对浸出效率的影响。在最佳条件下,由1PEG-400:1PTSA (D1)组成的DES对铅(Pb)具有较高的选择性增溶能力,回收率为100 %,金(Au)回收率为55 %,锌回收率仅为6 %。由2ChCl:1PTSA (D2)组成的DES对Pb和Zn均有较强的溶解能力,Pb和Zn的回收率均接近100% %,Au的回收率为73 %。最后,由1ChCl:2Acetic acid (D3)组成的DES对Pb的溶出效果也很好,回收率可达86 %以上,锌的回收率可达4 %。铅的溶解主要受扩散控制,活化能在24 ~ 34 kJ/mol之间。对于锌,较低的活化能22-32 kJ/mol表明锌处于扩散和化学控制之间的过渡状态。铅和锌的溶解均未超过40 kJ/mol的化学控制阈值。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


