Heterogeneity of TP53 mutations necessitates differentiation with p53-rescue therapies
IF 66.8
1区 医学
Q1 ONCOLOGY
引用次数: 0
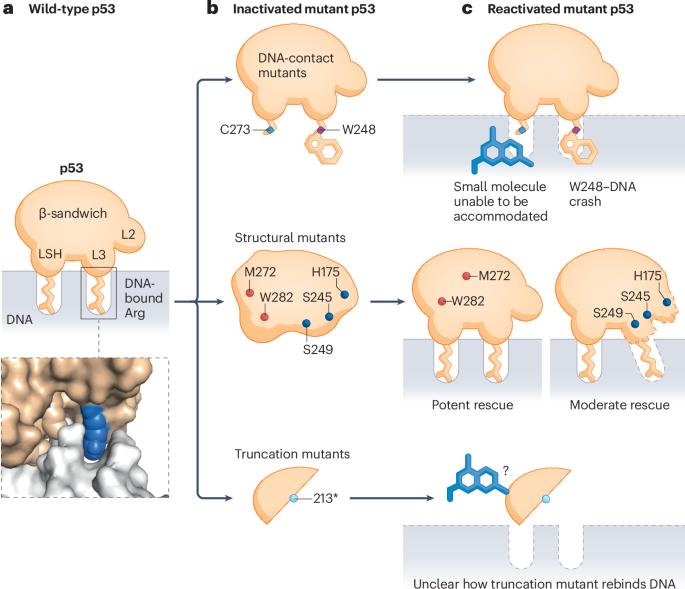

TP53突变的异质性需要区分p53拯救疗法
我们饶有兴趣地阅读了Peuget等人的评论(Peuget, S., Zhou, X. &;将基于p53的癌症治疗方法应用于临床。Nat. Rev. Cancer 24, 192-215(2024))1,介绍了基于p53的治疗方法的发展。我们完全同意p53靶向药物的巨大临床价值,因为TP53是癌症中最常见的突变基因1。然而,我们想指出的是,描述可以拯救两种或两种以上不同类型的p53突变体的小分子化合物需要非常谨慎。在23项已注册的p53拯救试验中,17项试验正在1,000多名癌症患者中研究这些化合物,而没有区分TP53突变(补充表1)。在过去的几十年里,人们一直在广泛研究结合突变型p53并恢复其肿瘤抑制功能的小分子化合物,至少发现了71种拯救化合物2,3,4。其中大多数被称为通用拯救化合物,可以同时拯救许多突变体,产生了数百个使用这些化合物拯救任意选择的p53突变体的基础和临床研究。然而,基于p53突变体不同作用机制的逻辑(图1b)和越来越多的实验验证[5,6,7],一个共识正在形成,即没有一种通用的拯救化合物可以提供“一贯性”的解决方案来拯救两种或两种以上类型的p53突变体。例如,p53-热稳定化合物可以将“展开-折叠”的动态平衡转向折叠,从而促进结构突变体的再折叠并拯救它们。因此,这种化合物在机制上和实验上都被证实对缺乏大片段氨基酸的p53截断突变体或缺乏dna结合氨基酸的dna接触突变体无效5,6,7。同样,dna接触的突变体拯救化合物(如果存在的话)应该通过补偿丢失的dna结合氨基酸来起作用,因此,从机制上讲,不能拯救未展开的结构突变体。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Reviews Cancer
医学-肿瘤学
CiteScore
111.90
自引率
0.40%
发文量
97
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Cancer, a part of the Nature Reviews portfolio of journals, aims to be the premier source of reviews and commentaries for the scientific communities it serves. The correct abbreviation for abstracting and indexing purposes is Nat. Rev. Cancer. The international standard serial numbers (ISSN) for Nature Reviews Cancer are 1474-175X (print) and 1474-1768 (online). Unlike other journals, Nature Reviews Cancer does not have an external editorial board. Instead, all editorial decisions are made by a team of full-time professional editors who are PhD-level scientists. The journal publishes Research Highlights, Comments, Reviews, and Perspectives relevant to cancer researchers, ensuring that the articles reach the widest possible audience due to their broad scope.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


